Abstract
Purpose
The evolutionary-conserved Wnt/β-CATENIN (WBC) pathway has been implicated in the pathogenesis of different solid malignant tumors. We evaluated the prognostic relevance of β-CATENIN, a pivotal mediator of WBC activation, in patients with human papillomavirus (HPV)-positive head and neck squamous cell carcinoma (HNSCC).
Methods
We analyzed if patients with HPV-positive HNSCC from the “The Cancer Genome Atlas” (TCGA cohort, n = 41) can be stratified based on their CTNNB1 mRNA expression. Moreover, in a tissue microarray (TMA) of primary tumor sections from HPV-positive HNSCC patients treated in a tertiary academic center (in-house cohort, n = 31), we evaluated the prognostic relevance of β-CATENIN expression on protein level.
Results
In silico mining of CTNNB1 expression in HPV-positive HNSCC revealed that high CTNNB1 expression was linked to better overall survival (OS, p = 0.062). Moreover, high β-CATENIN expression was significantly associated with a better OS in our in-house cohort (p = 0.035).
Conclusion
Based on these findings, we postulate that β-CATENIN expression could serve (potentially in conjunction with other WBC pathway members) as a marker for better survival outcomes in patients with HPV-positive HNSCC. However, it is evident that future studies on bigger cohorts are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-04712-3.
Keywords: β-Catenin; WnT/β-Catenin pathway, HPV; Head and neck cancer; Immunohistochemistry
Introduction
Head and neck squamous cell carcinoma (HNSCC) originate from the mucosal linings of the upper aerodigestive tract. In 2020, approximately 870,000 new cases were recorded, making HNSCC the seventh most common cancer worldwide (Sung et al. 2021). Habits, such as smoking and alcohol consumption, chronically expose the mucosa of the aerodigestive tract to environmental carcinogens, which are a major contributor to HNSCC tumorigenesis. Although the numbers of smokers and drinkers are globally gradually decreasing (Brkic et al. 2021), an increase of the relative incidence rate of oropharyngeal squamous cell carcinoma (OPSCC) has been observed. This is most likely linked to the rise in human papillomavirus (HPV) infections (Brkic et al. 2021). For the last 2 decades, the role and importance of the DNA oncovirus HPV in OPSCC has been well illustrated, particularly for the high-risk variant “HPV-16” (Elrefaey et al. 2014a; Ward et al. 2014). HPV has epitheliotropic properties, meaning it has an affinity for epithelial tissue (Elrefaey et al. 2014a). Especially the local crypts of the oropharynx facilitate its replication, hence there is an inherent propensity of HPV to infect the epithelium of the oropharynx (Elrefaey et al. 2014b; Elrefaey et al. 2014a). Despite HPV-positive OPSCC being linked to better survival outcomes, their clinical management does not differ from their HPV-negative counterparts. Until now, the most widely utilized systemic therapy is cisplatin which is highly effective but comes with severe side effects including acute kidney failure, alopecia, and neuropathy (Mehanna et al. 2019). Hence, recently options for treatment de-escalation have been discussed due to the comparatively favorable survival rates of HPV-positive OPSCC (Golusinski et al. 2021). Nonetheless, specific findings related to the identification of novel prognostic markers which could guide treatment decisions are scarce. Intriguingly, classical risk factors, such as T/N stage, do not have the same prognostic value in HPV-positive HNSCC as in HPV-negative HNSCC (Elrefaey et al. 2014b). Therefore, discovering novel markers for patient stratification at early stages might facilitate better patient management and identification of de-escalation treatment candidates.
Wnt/β-CATENIN (WBC) signaling is an important, well-conserved signaling pathway throughout evolution and is implicated in various key cellular processes, such as proliferation, migration, and cell differentiation (Lee et al. 2014; Pai et al. 2017). Due to its diverse roles, a deregulation of the WBC pathway can have profound consequences, such as carcinogenesis. This has already been illustrated for colorectal and ovarian cancer (Zhan et al. 2016). Recent evidence suggests that the activation of canonical WBC signaling plays a role in the disease progression of HPV-negative HNSCC by modulating the migratory and invasive potential of tumor cells (Moon et al. 2021). In contrast, the role of deregulated WBC signaling in HPV-positive HNSCC is still largely unknown.
Multiple lines of evidence suggest that the HPV-specific viral oncoproteins E6 and E7 directly or indirectly interact with β-CATENIN, thereby positively modulating its activity. For example, the viral oncoprotein E6 blocks the proteasomal degradation of β-CATENIN—promoting its nuclear translocation and activation of the pathway. This abnormal increase in WBC pathway activity leads to/can lead to the carcinogenesis of cervical cancer (Wang et al. 2020). Furthermore, Rampias et al. (2010) unveiled that the function of seven in absentia homologue (Siah-1), which can promote the degradation of β-CATENIN, is repressed by the viral oncoproteins E6 and E7. This repression of Siah-1 by E6 and E7 indicates their direct role in the nuclear accumulation of β-CATENIN in HPV-associated OPSCC. Interestingly, blockade of the WBC pathway by a specific inhibitor of Creb-binding protein (CBP) has been demonstrated to be specifically effective in the HPV-positive HNSCC cell line SCC154 compared to the HPV-negative HNSCC cell line Cal27 (Brkic et al. 2022). Moreover, MSAB, a small inhibitor of β-CATENIN, has been proven to have antineoplastic effects in HNSCC as well—supporting the oncogenic role of the WBC pathway in HNSCC (Maier et al. 2023).
Based on this status quo, we aimed to investigate the association of WBC signaling with survival in HPV-positive HNSCC. In particular, we aimed to assess the prognostic potential of β-CATENIN expression in two independent cohorts in silico, on mRNA and protein level. These results could potentially facilitate the identification of a new and easily obtainable prognostic marker for HPV-positive HNSCC.
Materials and methods
The cancer genome atlas (TCGA)—TCGA cohort
For the analysis of the prognostic value of CTNNB1 expression, data from all eligible HPV-positive HNSCC patients (= positive status for HPV via fluorescence in situ hybridization (FISH) or positive status for p16 staining) was retrieved using the TCGA database (n = 41, https://portal.gdc.cancer.gov/projects/TCGA-HNSC. Accessed on 1st of April 2022). All available clinical, follow-up and gene expression (Illumina HiSeq RNA sequencing) data from patients with a primary treated and HPV-positive HNSCC were retrieved from the GDC Legacy Archive (https://portal.gdc.cancer.gov/legacy-archive/search/f. Accessed on 30th of December 2021) and normalized utilizing the R package “TCGA biolinks” (version 2.19.0) and R (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria). Furthermore, the expression values of CTNNB1 for all 41 patients were filtered and used for the survival analysis (see Statistical analysis section for further details).
Immunohistochemistry (IHC)—the in-house cohort
Patients with a histologically verified HPV-positive HNSCC, primarily surgically treated in the General Hospital of Vienna between 1st of January 2012 and 31st of December 2017, were included in this investigation. The HPV positivity was determined by FISH. The patients which did not match the inclusion criteria (no recurrent disease and no secondary malignancy) were not considered for the analysis. Besides the tissue, data on age, sex, tumor staging, and other clinically relevant data were retrospectively collected.
For the analysis of the association of β-CATENIN expression with overall survival (OS) and disease-free survival (DFS), a tissue microarray (TMA) of formalin-fixed paraffin-embedded (FFPE) biopsy samples of all eligible patients was constructed. The construction of the TMA was performed with the computer-assisted tissue microarray platform (TMA Grand Master, 3D Histech, Budapest, Hungary). Areas in the FFPE biopsy samples containing predominantly tumor cells were chosen as source material for the TMA cores (general layout of the TMA is shown in supplementary Fig. 1). The immunohistochemical staining was performed on 4 µm slices with the Lab Vision UltraVision Kit (Thermo Scientific, TL-060-HL). Colon tissue sections served as a positive control for the staining. The actual staining was performed as previously described (Brkic et al. 2022) with a 1:50 dilution of the β-CATENIN antibody (Santa Cruz, sc-7963). For the quantification of positive β-CATENIN staining QuPath (Version 0.2.3) was used and the percentage (0–100%) of stained cells for each of the patient TMA cores (3 replicates per patient) was analyzed. Next, the mean of all three cores per patient was calculated and used in the subsequent survival analysis (see Statistical analysis section for further details).
Statistical anaylsis
The log-rank test for the survival analyses (TCGA cohort and in-house cohort) was performed utilizing the R packages “survival” and “survminer” (versions 3.2.13 and 0.4.9, respectively) and a p value below 0.05 was considered as statistically significant. The Kaplan–Meier curves were plotted with “ggplot” (version 3.3.3).
For the patient stratification of the TCGA cohort, the optimized threshold value (OTV) of CTNNB1 expression regarding OS was calculated using the surv_cutpoint function from the “survminer” R package with a patient distribution of at least 20% per group and resulted in a value of 15,943. Based on this value, the patients were stratified and the resulting patient distribution was plotted in a Kaplan–Meier curve.
For the patient stratification of the in-house cohort the OTVs, with regards to OS and DFS, were calculated (surv_cutpoint function from the “survminer” R package) and used as a cutoffs (> OTV was considered as high). The calculation of the OTV with a patient distribution of at least 20% per group resulted in a cutoff of 7.33%, for OS and DFS, respectively. We plotted the Kaplan-Meier curves with “ggplot”.
Results
High expression of CTNNB1 mRNA is associated with a better overall survival—TCGA cohort
CTNNB1 expression and clinical data of 41 HPV-positive HNSCC patients were retrieved from the TCGA database. The characteristics of the whole cohort, as well as the subgroups—post-stratification based on the OTV of CTNNB1 expression—are shown in Table 1.
Table 1.
Patient characteristics of HPV-positive HNSCC patients of the TCGA cohort—stratified by CTNNB1 expression
| Characteristic | CTNNB1 low | CTNNB1 high | Total |
|---|---|---|---|
| Number of patients (%) | 23 | 18 | 41 |
| Age, median (years) | 57.4 | 53.5 | 57.0 |
| Range (years) | 41.1–68.3 | 40.6–71.5 | 40.6–71.5 |
| T stage | n (%) | n (%) | n (%) |
| T4 | 6 (14.6) | 2 (4.9) | 8 (19.5) |
| T3 | 3 (7.3) | 2 (4.9) | 5 (12.2) |
| T2 | 12 (29.3) | 9 (21.9) | 21 (51.2) |
| T1 | 2 (4.9) | 4 (9.8) | 6 (14.7) |
| Tx | 0 (0.0) | 1 (2.4) | 2 (2.4) |
| N stage | n (%) | n (%) | n (%) |
| N3 | 0 (0.0) | 1 (2.4) | 1 (2.4) |
| N2 | 16 (39.0) | 10 (24.4) | 26 (63.4) |
| N1 | 2 (4.9) | 3 (7.3) | 5 (12.2) |
| N0 | 4 (9.8) | 4 (9.8) | 8 (19.6) |
| Nx | 0 (0.0) | 1 (2.4) | 1 (2.4) |
| M stage | n (%) | n (%) | n (%) |
| M0 | 21 (51.2) | 17 (41.5) | 38 (92.7) |
| Mx | 2 (4.9) | 1 (2.4) | 3 (7.3) |
The patient characteristics and the distribution into the two groups post-stratification based on CTNNB1 expression are shown.
TCGA The Cancer Genome Atlas.
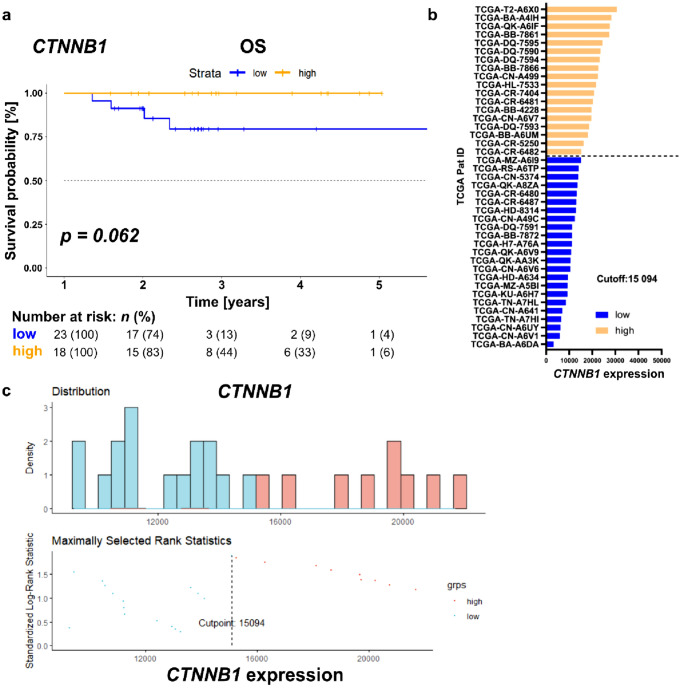
After stratification by the OTV of CTNNB1, high CTNNB1 expression showed a trend of being associated with longer OS (Fig. 1, median OS for both groups not reached, 95% confidence interval (CI) not applicable, log-rank p = 0.062). The distribution of the patients across the two groups is shown in Fig. 1b, c.
Fig. 1.
CTNNB1 expression allows the identification of HPV-positive HNSCC patients with longer overall survival. a Kaplan–Meier survival curve for patients with HPV-positive HNSCC extracted from the TCGA database and stratified according to the OTV of CTNNB1 into “low” and “high” group. b Respective CTNNB1 expression values per patient stratified into the two groups (“low” [blue] and “high” [orange]) according to the OTV (indicated as “Cutoff”) for OS. c Top—Distribution of CTNNB1 expression in the two patient groups Bottom—Standardized Log-Rank statistics across multiple CTNNB1 expression values, indicating that a cutoff of 15,094 yields the highest prognostic power. HPV human papillomavirus, TCGA The Cancer Genome Atlas, OTV optimized threshold value, grps groups
High β-CATENIN expression predicts a better overall survival—in-house cohort
Based on the in silico analysis of CTNNB1 expression in HPV-positive HNSCC patients of the TCGA cohort, we sought to evaluate the expression levels and prognostic value of β-CATENIN on protein level. A collection of HPV-positive HNSCC cases, primarily surgically treated at the General Hospital of Vienna, was screened. A total of 31 patients with available follow-up fulfilled the inclusion criteria. Table 2 illustrates the detailed patient characteristics of the in-house cohort. Thirteen patients were female (41.9%). The median age of the cohort was 64 years (range 37.0–80.5 years). A small subset of patients presented with advanced local disease (T3-4, n = 5, 16.1%). Furthermore, none of the patients had distant metastases during the initial work-up. All patients underwent primary surgical resection, from which 23 patients (74.2%) received post-operative radiotherapy (PORT). The median OS and DFS for all patients were 1.8 years (range 0.3–12.3 years) and 1.5 years (range 0.0–9.8 years), respectively.
Table 2.
Patient and tumor characteristics of the in-house cohort
| Characteristic | Number of patients | Percentage (%) |
|---|---|---|
| Gender | ||
| Female | 13 | 41.9 |
| Male | 18 | 58.1 |
| T stage | ||
| T4 | 4 | 12.9 |
| T3 | 1 | 3.2 |
| T2 | 17 | 54.9 |
| T1 | 9 | 29 |
| N stage | ||
| N3 | 1 | 3.2 |
| N2 | 15 | 48.4 |
| N1 | 10 | 32.3 |
| N0 | 5 | 16.1 |
| M stage | ||
| M0 | 31 | 100 |
| Grading | ||
| G3 | 10 | 32.3 |
| G2 | 20 | 64.5 |
| G1 | 1 | 3.2 |
| PORT | ||
| Yes | 23 | 74.2 |
| No | 8 | 25.8 |
PORT post-operative radiotherapy
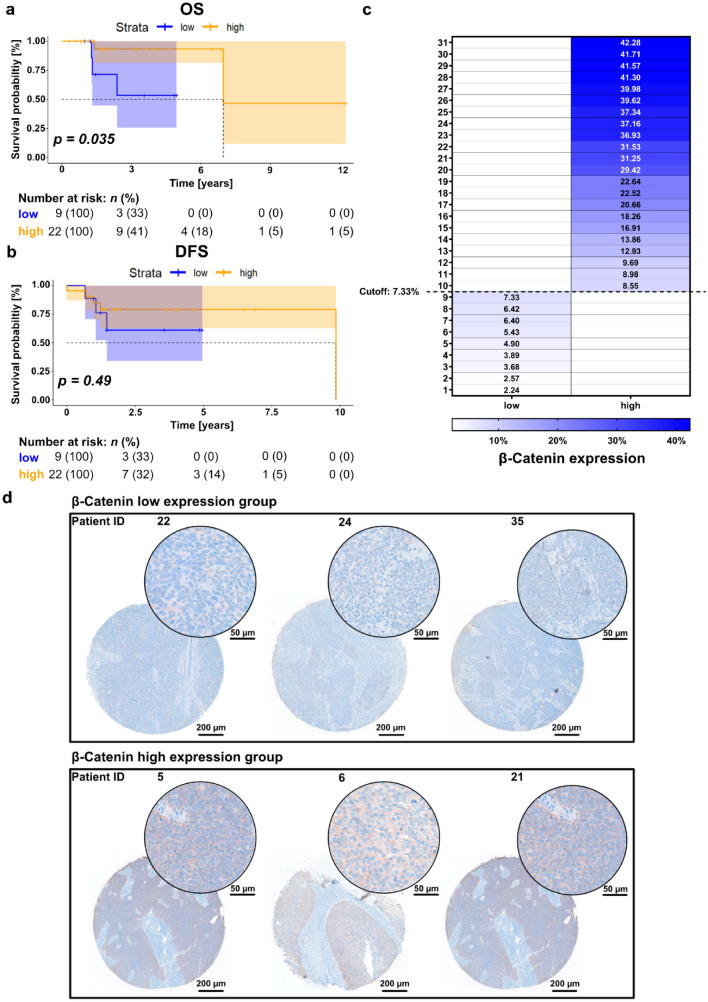
Similar to the TCGA analysis on mRNA level, longer OS times were observed in the group with high β-CATENIN expression with the OTV as cutoff (Fig. 2a, median OS for low group not reached, median OS for high group: 6.97 years, log-rank p = 0.035). However, an association between β-CATENIN expression and DFS was not observed (Fig. 2a, median OS for low group not reached, median OS for high group: 9.84 years, log-rank p = 0.49). The distribution of the percentage of cells showing positive staining and their relation to the two groups (stratification based on OTV) is shown in Fig. 2c. Figure 2d shows representative images of the IHC staining for three patients per group at two different magnifications.
Fig. 2.
β-CATENIN is heterogeneously expressed in cancer samples of HPV-positive HNSCC and predicts favorable overall survival. a, b Kaplan–Meier survival curve for OS (a) and DFS (b) stratified into low and high expression according to the OTV of β-CATENIN expression. 95% confidence intervals are visualized by the shaded areas in the Kaplan–Meier survival curves. c Heatmap illustrating the percentage of cells positive for β-CATENIN expression per patient stratified into the two groups. “Low” (left column) and “high” (right column) expression were stratified by the OTV (indicated as “Cutoff” in the figure). d Representative IHC images for patients with “low” (top row) and “high” (bottom row) β-CATENIN expression according to the OTV. The image of the whole TMA core was acquired at a 5 × magnification and the close-up was acquired at a 20 × magnification. OS overall survival, DFS disease-free survival, OTV optimized threshold value, IHC immunohistochemistry staining, TMA tissue microarray
Discussion
In the present study, we provided novel evidence that high CTNNB1 expression was associated with better OS in patients with HPV-associated HNSCC. Furthermore, high β-CATENIN protein levels were associated with a favorable OS in an independent in-house cohort. Hence, β-CATENIN expression could facilitate the identification of HPV-positive HNSCC patients with a better prognosis, potentially providing an easily available tool for identification of de-escalation treatment candidates. β-CATENIN is a pivotal component and positive regulator of the WBC pathway, and its prognostic value has been demonstrated in various cancer entities (Ellison et al. 2005; Kamposioras et al. 2013; Nagy et al. 2017). However, reports of its prognostic value in HNSCC patients are rare, particularly for HPV-positive HNSCC. Therefore, we evaluated whether HPV-positive HNSCC patients can be effectively stratified based on their CTNNB1/β-CATENIN expression. Interestingly, our analysis revealed that high CTNNB1/β-CATENIN expression is associated with better OS times. Similarly, high β-CATENIN was shown to associate with a lower risk of death in pancreatic cancer (Saukkonen et al. 2016) and in surgically treated colorectal patients, Kamposioras et al. (Kamposioras et al. 2013) found a positive association between β-CATENIN expression and DFS. High viral load is associated with better survival in patients with HPV-associated cancers. This fact might explain why β-CATENIN, which expression is modulated by the viral oncoproteins E6 and E7, has a similar prognostic effect (Deng et al. 2015; Hashida et al. 2021). Specifically, Rampias et al. showed that β-CATENIN expression is positively regulated by the viral oncoproteins E6 and E7. Hence, a high viral load might lead to an E6- and E7-mediated increase in β-CATENIN expression. Despite this evidence, further studies investigating the exact mechanistic nature of these associations are needed.
As described previously, patients with HPV-positive OPSCC generally have a good prognosis. Therefore, strategies for treatment de-escalation have been investigated (Mirghani and Blanchard 2018; Bonomo and Livi 2020). These treatment de-escalation regimes should decrease treatment-associated morbidity while maintaining comparable efficacy. Since CTNNB1/β-CATENIN was overexpressed in patients with good prognosis, potential therapies tailored toward β-CATENIN-expressing cells, such as PRI-724 (a small molecule inhibitor of the interaction between CBP and β-CATENIN), might be a viable option for treatment de-escalation for these HPV-positive HNSCC patients (Zhang and Wang 2020).
The limited size of the TCGA and the in-house cohort are evident limitations of the study. This is attributable to the circumstance that only about 25% of all diagnosed HNSCC cases are associated with HPV (Tumban 2019; Dong et al. 2021). Nonetheless, a trend toward a significant association of high CTNNB1 expression and a better survival outcome was observed in the TCGA cohort. Furthermore, the hypothesis that high β-CATENIN expression is associated with a better OS could be validated on protein level in our independent in-house cohort. Lastly, there are currently no standardized methods for differentiation between active and latent HPV infection in HNSCC. However, as the HPV positivity was shown by FISH, we can hypothesize that the HPV infection was active in all used tissue samples (Gkolfinopoulos et al. 2021).
Especially due to the above-mentioned weak point of the study, further investigations (including functional assessments of the role of β-CATENIN in HPV-positive HNSCC and studies exploring the prognostic value of β-CATENIN in larger patient cohorts) on the prognostic value of β-CATENIN expression are recommended. This could enable the establishment of an additional prognostic marker (in addition to the HPV status), which would facilitate timely risk stratification of HPV-associated HNSCC patients and ultimately facilitate identifying de-escalation treatment candidates.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- CBP
Creb-binding protein
- DFS
Disease-free survival
- FFPE
Formalin-fixed paraffin-embedded
- HNSCC
Head and neck squamous cell carcinoma
- HPV
Human papillomavirus
- IHC
Immunohistochemistry
- OPSCC
Oropharyngeal squamous cell carcinoma
- OS
Overall survival
- OTV
Optimized threshold value
- PORT
Postoperative radiotherapy
- Siah-1
Seven in absentia homologue
- TCGA
The Cancer Genome Atlas
- TMA
Tissue microarray
- WBC
Wnt/β-Catenin
Author contributions
Study concept, SS, FFB and LK-W; study design SS, FFB; data acquisition, SS, FFB, JS and EG; quality control of data and algorithms, FFB and LK-W; data analysis and interpretation, SS, FFB and TM; statistical analysis, SS, FFB; manuscript preparation, SS; manuscript preparation—editing, SS, FFB, JS, TM, LK, LK-W and GH; manuscript preparation—review, SS, FFB, JS, TM, EG, LK, LK-W and GH. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by Medical University of Vienna. F.F.B. has received research grants from the Ph.D. Martina Hamböck Grant of the Vienna Medical Chamber (Grant Number 0007-WS 2020), as well as from the Medical Scientific Fund of the Mayor of the city of Vienna (Grant Number 19066).
Data availability
The public dataset supporting the conclusions of this article is available in the TCGA repository [TCGA-HNSC, https://portal.gdc.cancer.gov/projects/TCGA-HNSC].
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
The study approval for the IHC analysis and retrospective data sampling of patients treated in our center was obtained from the ethics committee of the Medical University of Vienna (Approval Number EK1262/2019). For the analysis of the publicly available TCGA data, no ethics approval was needed according to the guidelines of our institution.
Consent for publication
Not applicable.
Conflict of interest
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stefan Stoiber and Faris F. Brkic have contributed equally.
Lorenz Kadletz-Wanke and Lukas Kenner have contributed equally.
Contributor Information
Stefan Stoiber, Email: stefan.stoiber@meduniwien.ac.at.
Faris F. Brkic, Email: faris.brkic@meduniwien.ac.at
Tobias Maier, Email: tobias.maier@meduniwien.ac.at.
Julia Schnoell, Email: julia.schnoell@meduniwien.ac.at.
Elisabeth Gurnhofer, Email: elisabeth.gurnhofer@meduniwien.ac.at.
Gregor Heiduschka, Email: gregor.heiduschka@meduniwien.ac.at.
Lorenz Kadletz-Wanke, Email: lorenz.kadletz-wanke@meduniwien.ac.at.
Lukas Kenner, Email: lukas.kenner@meduniwien.ac.at.
References
- Bonomo P, Livi L. De-intensification for HPV positive oropharyngeal cancer: and yet it moves!: 2019 in review. Clin Transl Radiat Oncol. 2020;22:40–43. doi: 10.1016/J.CTRO.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkic FF, et al. An analysis of distant metastasis cases from HPV-associated oropharyngeal squamous cell carcinoma. J Craniomaxillofac Surg. 2021;49(4):312–316. doi: 10.1016/J.JCMS.2021.01.012. [DOI] [PubMed] [Google Scholar]
- Brkic FF, et al. Targeting Wnt/beta-catenin signaling in HPV-positive head and neck squamous cell carcinoma. Pharmaceuticals. 2022;15(3):378. doi: 10.3390/PH15030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T, et al. Low initial human papillomavirus viral load may indicate worse prognosis in patients with cervical carcinoma treated with surgery. J Gynecol Oncol. 2015;26(2):111–117. doi: 10.3802/JGO.2015.26.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, et al. Current status of human papillomavirus-related head and neck cancer: from viral genome to patient care. Virol Sin. 2021;36(6):1284–1302. doi: 10.1007/S12250-021-00413-8/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison DW, et al. β-catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom children’s cancer study group brain tumour committee. J Clin Oncol. 2005;23(31):7951–7957. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- Elrefaey S, et al. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299. [PMC free article] [PubMed] [Google Scholar]
- Gkolfinopoulos S, Economopoulou P, Psyrri A. Prognostic role of p16/HPV in non-oropharyngeal head and neck squamous cell cancer (HNSCC) Crit Issues Head Neck Oncol. 2021 doi: 10.1007/978-3-030-63234-2_12. [DOI] [Google Scholar]
- Golusinski P, et al. De-escalation studies in HPV-positive oropharyngeal cancer: how should we proceed? Oral Oncol. 2021;123:105620. doi: 10.1016/J.ORALONCOLOGY.2021.105620. [DOI] [PubMed] [Google Scholar]
- Hashida Y, et al. Prognostic significance of human papillomavirus 16 viral load level in patients with oropharyngeal cancer. Cancer Sci. 2021;112(10):4404. doi: 10.1111/CAS.15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamposioras K, et al. The prognostic significance of WNT pathway in surgically-treated colorectal cancer: β-catenin expression predicts for disease-free survival. Anticancer Res. 2013;33(10):4573–4584. [PubMed] [Google Scholar]
- Lee SH, et al. Wnt/β-catenin signalling maintains self-renewal and tumourigenicity of head and neck squamous cell carcinoma stem-like cells by activating Oct4. J Pathol. 2014;234(1):99–107. doi: 10.1002/PATH.4383. [DOI] [PubMed] [Google Scholar]
- Maier T, et al. Inhibition of beta-catenin shows therapeutic potential in head and neck squamous cell carcinoma in vitro. Eur Arch Otorhinolaryngol. 2023;280(1):399–408. doi: 10.1007/S00405-022-07598-Y/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna H, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393(10166):51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2018;8:4. doi: 10.1016/J.CTRO.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JH, Lee SH, Lim YC. Wnt/β-catenin/slug pathway contributes to tumor invasion and lymph node metastasis in head and neck squamous cell carcinoma. Clin Exp Metastasis. 2021;38(2):163–174. doi: 10.1007/S10585-021-10081-3. [DOI] [PubMed] [Google Scholar]
- Nagy B, et al. Nuclear β-catenin positivity as a predictive marker of long-term survival in advanced epithelial ovarian cancer. Pathol Res Pract. 2017;213(8):915–921. doi: 10.1016/J.PRP.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Pai SG, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):1–12. doi: 10.1186/S13045-017-0471-6/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampias T, et al. Activation of Wnt signaling pathway by human papillomavirus E6 and E7 oncogenes in HPV16-positive oropharyngeal squamous carcinoma cells. Mol Cancer Res. 2010;8(3):433–443. doi: 10.1158/1541-7786.MCR-09-0345/356856/P/ACTIVATION-OF-WNT-SIGNALING-PATHWAY-BY-HUMAN. [DOI] [PubMed] [Google Scholar]
- Saukkonen K, et al. PROX1 and β-catenin are prognostic markers in pancreatic ductal adenocarcinoma. BMC Cancer. 2016;16(1):1–12. doi: 10.1186/S12885-016-2497-5/TABLES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, et al. Global cancer statistics 2020 globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/CAAC.21660. [DOI] [PubMed] [Google Scholar]
- Tumban E. A current update on human papillomavirus-associated head and neck cancers. Viruses. 2019;11(10):922. doi: 10.3390/V11100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, et al. β-Catenin: oncogenic role and therapeutic target in cervical cancer. Biol Res. 2020;53(1):1–11. doi: 10.1186/S40659-020-00301-7/METRICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MJ, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489. doi: 10.1038/BJC.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2016;36(11):1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):1–16. doi: 10.1186/S13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The public dataset supporting the conclusions of this article is available in the TCGA repository [TCGA-HNSC, https://portal.gdc.cancer.gov/projects/TCGA-HNSC].