Abstract
Aims
The HeartLogic algorithm combines multiple implantable defibrillator (ICD) sensor data and has proved to be a sensitive and timely predictor of impending heart failure (HF) decompensation in cardiac resynchronization therapy (CRT‐D) patients. We evaluated the performance of this algorithm in non‐CRT ICD patients and in the presence of co‐morbidities.
Methods and results
The HeartLogic feature was activated in 568 ICD patients (410 with CRT‐D) from 26 centres. The median follow‐up was 26 months [25th–75th percentile: 16–37]. During follow‐up, 97 hospitalizations were reported (53 cardiovascular) and 55 patients died. We recorded 1200 HeartLogic alerts in 370 patients. Overall, the time IN the alert state was 13% of the total observation period. The rate of cardiovascular hospitalizations or death was 0.48/patient‐year (95% CI: 0.37–0.60) with the HeartLogic IN the alert state and 0.04/patient‐year (95% CI: 0.03–0.05) OUT of the alert state, with an incidence rate ratio of 13.35 (95% CI: 8.83–20.51, P < 0.001). Among patient characteristics, atrial fibrillation (AF) on implantation (HR: 1.62, 95% CI: 1.27–2.07, P < 0.001) and chronic kidney disease (CKD) (HR: 1.53, 95% CI: 1.21–1.93, P < 0.001) independently predicted alerts. HeartLogic alerts were not associated with CRT‐D versus ICD implantation (HR: 1.03, 95% CI: 0.82–1.30, P = 0.775). Comparisons of the clinical event rates in the IN alert state with those in the OUT of alert state yielded incidence rate ratios ranging from 9.72 to 14.54 (all P < 0.001) in all groups of patients stratified by: CRT‐D/ICD, AF/non‐AF, and CKD/non‐CKD. After multivariate correction, the occurrence of alerts was associated with cardiovascular hospitalization or death (HR: 1.92, 95% CI: 1.05–3.51, P = 0.036).
Conclusions
The burden of HeartLogic alerts was similar between CRT‐D and ICD patients, while patients with AF and CKD seemed more exposed to alerts. Nonetheless, the ability of the HeartLogic algorithm to identify periods of significantly increased risk of clinical events was confirmed, regardless of the type of device and the presence of AF or CKD.
Keywords: Atrial fibrillation, Chronic kidney disease, CRT, Heart failure, ICD, Risk stratification
Introduction
Implantable cardioverter defibrillators (ICD) and defibrillators for resynchronization therapy (cardiac resynchronization therapy defibrillator [CRT‐D]) are widely adopted for the management of chronic heart failure (HF). 1 , 2 , 3 Some modern ICDs are equipped with automated algorithms that provide detailed information on the HF condition on a daily basis. Many studies have investigated the ability of ICD diagnostics to identify patients at risk of HF events, with contradictory results. 4 , 5 , 6 , 7 In the past decade, studies have reported combining ICD diagnostics in order to better stratify and manage patients at risk of HF events. 8 , 9 , 10 In the Multisensor Chronic Evaluation in Ambulatory Heart Failure Patients (MultiSENSE) study, 11 a novel algorithm for HF monitoring was implemented: the HeartLogic (Boston Scientific, St. Paul, Minnesota) Index, which combines physiological data from multiple ICD‐based sensors. The index enabled dynamic assessment of HF, identifying periods when patients were at significantly increased risk of worsening HF. 12 However, the algorithm was developed only on the basis of data from CRT‐D patients, and there are no data on its performance in the presence of cardiovascular and non‐cardiovascular co‐morbidities, which are common in HF patients and are known to affect disease severity and prognosis. In the present study, we sought to evaluate the incidence of HeartLogic alerts in patients who received either CRT‐D or ICD, and to investigate the impact of co‐morbidities on the frequency of alerts and on the risk stratification ability of the algorithm.
Methods
Patient selection
The study was a prospective, non‐randomized, multicentre evaluation of patients who had received an ICD or cardiac resynchronization therapy ICD (CRT‐D) endowed with the HeartLogic™ diagnostic algorithm. Consecutive HF patients with reduced left ventricular ejection fraction (≤35% at the time of implantation) who had received a device in accordance with standard indications 1 and were enrolled in the LATITUDE (Boston Scientific) remote monitoring platform were included at 26 study centres (full list of participating centres is in Data S1) and followed up in accordance with the standard practice of the participating centres. Clinics periodically checked the remote monitoring website for transmissions. Moreover, remote data reviews and patient phone contacts were undertaken at the time of HeartLogic alerts, to assess the patient's decompensation status and, if possible, to prevent further worsening. However, the study protocol did not mandate any specific intervention algorithm and physicians were free to remotely implement clinical actions, or to schedule extra in‐office visits when deemed necessary. Data on the clinical events that occurred during follow‐up were collected at the study centres within the framework of a prospective registry (ClinicalTrials.gov identifier: NCT02275637). The Institutional Review Boards approved the study, and all patients provided written informed consent for data storage and analysis.
Device characteristics
Commercially available ICD/CRT‐Ds equipped with the HeartLogic™ diagnostic feature and standard transvenous leads were used in this study. The details of the HeartLogic algorithm have been reported previously. 11 Briefly, the algorithm combines data from multiple sensors: accelerometer‐based first and third heart sounds, intrathoracic impedance, respiration rate, the ratio of respiration rate to tidal volume, night heart rate, and patient activity. Each day, the device calculates the degree of worsening in sensors from their moving baseline and computes a composite index. An alert is issued when the index crosses a programmable threshold (nominal value, 16). When the index enters an alert state, the ‘exit‐alert’ threshold is automatically dropped to a recovery value (nominal value, 6).
Study endpoints
The objectives of the present analysis were to compare the incidence of HeartLogic alerts between CRT and non‐CRT patients, and to evaluate their frequency in relation to the presence of co‐morbidities. HeartLogic index values >16 identified periods as IN an alert state versus OUT of an alert state. We also investigated the impact of the presence of co‐morbidities on the performance of the HeartLogic Index in detecting follow‐up periods of significantly increased risk of clinical events. The study endpoints consisted of cardiovascular hospitalizations requiring at least one overnight stay and the combination of cardiovascular hospitalizations and death.
Statistical analysis
Quantitative variables are reported as means±SD if normally distributed, or medians with 25th to 75th percentiles in the case of skewed distribution. Normality of distribution was tested by means of the nonparametric Kolmogorov–Smirnov test. Categorical data are expressed as percentages. Differences between mean data were compared by means of a t‐test for Gaussian variables, and Mann–Whitney or Wilcoxon non‐parametric test for non‐Gaussian variables, respectively, for independent or paired samples. Differences in proportions were compared by means of χ 2 analysis. Clinical event rates were calculated separately during IN and OUT alert states in terms of the ratio between the total count of events occurring in each state and the respective patient follow‐up durations, and were expressed as events per patient‐year. Analysis of the time to the first event was made by means of the Kaplan–Meier method. Cox proportional hazards models were used to determine the association between patients' baseline characteristics and the occurrence of events during the follow‐up period, and to estimate the hazard ratios (HRs) and the 95% confidence intervals (CIs) of an episode. Additionally, the sensitivity (with 95% CI) for detecting cardiovascular hospitalizations and the combined endpoint of hospitalization or death were also computed by classifying alerts as true‐positive if the alert onset occurred and did not reset before an endpoint. Moreover, the false‐positive rate was defined as the ratio of the total number of alerts that were not true‐positive alerts over the total usable follow‐up duration. All variables displaying statistical significance (P‐value <0.05) were entered into a multivariate regression analysis. The time course of HeartLogic index and sensor changes surrounding the alert were evaluated at two time‐points. 13 A 30‐day baseline (average calculated 30 days prior to the alert) was compared with the alert state (weekly average calculated from the alert day to day 6). Sensor data were compared between different temporal periods by means of a paired t‐test. A P value <0.05 was considered significant for all tests. All statistical analyses were performed by means of R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
From December 2017 to June 2021, HeartLogic was activated in 568 patients who had received an ICD (n = 158) or CRT‐D (n = 410). The index threshold was programmed to the nominal value of 16 in all patients and was not modified during follow‐up. Table 1 shows the baseline clinical variables of all patients in the present analysis.
Table 1.
Demographics and baseline clinical parameters of the study population
| Parameter | Total (N = 568) | No alerts (N = 198) | Time in alert <20% (N = 219) | Time in alert ≥20% (N = 151) |
|---|---|---|---|---|
| Male gender, n (%) | 453 (80) | 158 (80) | 171 (78) | 124 (82) |
| Age, years | 69 ± 10 | 68 ± 9 | 69 ± 10 | 71 ± 11* |
| Ischaemic aetiology, n (%) | 285 (50) | 95 (48) | 103 (47) | 87 (58)# |
| NYHA class | # | |||
| Class I, n (%) | 36 (6) | 16 (8) | 16 (7) | 4 (3) |
| Class II, n (%) | 351 (62) | 126 (64) | 131 (60) | 94 (62) |
| Class III, n (%) | 171 (30) | 52 (26) | 71 (32) | 48 (32) |
| Class IV, n (%) | 10 (2) | 4 (2) | 1 (0.5) | 5 (3) |
| LV ejection fraction, % | 32 ± 9 | 33 ± 9 | 32 ± 9 | 30 ± 8 |
| AF history, n (%) | 196 (35) | 38 (19) | 78 (36)* | 80 (53)*, # |
| AF on implantation, n (%) | 100 (18) | 14 (7) | 42 (19)* | 44 (29)*, # |
| Diabetes, n (%) | 167 (29) | 52 (26) | 71 (32) | 44 (29) |
| COPD, n (%) | 89 (16) | 29 (15) | 32 (15) | 28 (19) |
| Chronic kidney disease, $ n (%) | 153 (27) | 33 (17) | 63 (29)* | 57 (38)* |
| Hypertension, n (%) | 334 (59) | 118 (60) | 128 (58) | 88 (58) |
| Beta‐blocker use, n (%) | 520 (92) | 185 (93) | 196 (89) | 139 (92) |
| ACE‐I, ARB or ARNI use, n (%) | 536 (94) | 186 (94) | 211 (96) | 139 (92) |
| Diuretic use, n (%) | 506 (89) | 167 (84) | 193 (88) | 146 (97)*, # |
| Antiarrhythmic use, n (%) | 116 (20) | 22 (11) | 49 (22)* | 45 (30)* |
| CRT device, n (%) | 410 (72) | 137 (69) | 162 (74) | 111 (74) |
| Primary prevention, n (%) | 500 (88) | 181 (91) | 187 (85) | 132 (87) |
Patients are stratified according to the percentage of time in alert during follow‐up.
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor‐neprilysin inhibitor; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; LV, left ventricular; NYHA, New York Heart Association.
P < 0.05 versus no alerts.
P < 0.05 versus <20%.
Estimated glomerular filtration rate <60 mL/min/1.73 m2.
Follow‐up
The median follow‐up was 26 months [25th–75th percentile: 16–37]. During the observation period, 97 hospitalizations were reported (53 for cardiovascular reasons) and 55 patients died. The HeartLogic index crossed the threshold value 1200 times (0.71 alerts/patient‐year) in 370 patients. The time in the IN‐alert state was 13% of the total observation period in the overall population and 20% of the follow‐up period of the 370 patients with alerts. Table 1 shows the clinical variables of patients stratified by time in the IN‐alert state (no alerts, <20%, ≥20%).
Association between HeartLogic alerts and baseline variables
Figure 1 shows the Kaplan–Meier analysis of time from implantation to the first HeartLogic alert in patients with CRT‐D and ICD (HR: 1.03, 95% CI: 0.82–1.30; P = 0.775). The results of the regression analysis of variables associated with alert occurrence are shown in Table 2 . Among patient characteristics, chronic kidney disease (CKD) (HR: 1.53, 95% CI: 1.21–1.93, P < 0.001) and atrial fibrillation (AF) on implantation (HR: 1.62, 95% CI: 1.27–2.07, P < 0.001) independently predicted alerts. The Kaplan–Meier plot of the time to the first alert in patients stratified according to CKD and AF on implantation is shown in Figure 1 .
Figure 1.
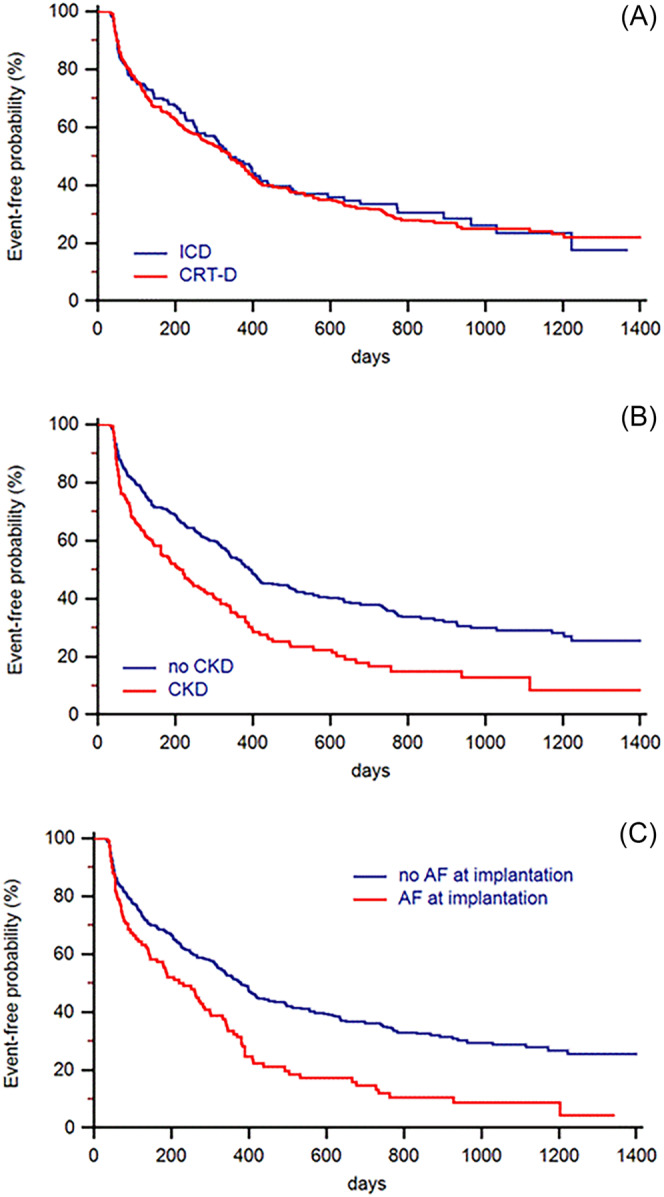
Kaplan–Meier analysis of time to first HeartLogic alert. Patients are stratified according to device type (CRT‐D: HR: 1.03, 95% CI: 0.82–1.30; P = 0.775) (A), chronic kidney disease at baseline (HR: 1.71; 95% CI: 1.33–2.18; P < 0.001) (B), and atrial fibrillation on implantation (HR: 1.77, 95% CI: 1.33–2.37; P < 0.001) (C).
Table 2.
Univariate and multivariate analysis of baseline variables associated with HeartLogic alert
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.02 | 1.00–1.03 | 0.006 | 1.01 | 0.99–1.02 | 0.210 |
| Male gender | 0.89 | 0.69–1.15 | 0.368 | ‐ | ‐ | ‐ |
| NYHA class | 1.08 | 0.91–1.27 | 0.405 | ‐ | ‐ | ‐ |
| Ischaemic heart disease | 1.23 | 1.00–1.51 | 0.047 | 1.06 | 0.85–1.32 | 0.589 |
| Ejection fraction | 0.99 | 0.98–1.01 | 0.242 | ‐ | ‐ | ‐ |
| History of AF | 1.81 | 1.47–2.22 | <0.001 | ‐ | ‐ | ‐ |
| AF on implantation | 1.78 | 1.40–2.27 | <0.001 | 1.62 | 1.27–2.07 | <0.001 |
| Hypertension | 0.97 | 0.79–1.19 | 0.766 | ‐ | ‐ | ‐ |
| Pulmonary disease | 1.09 | 0.82–1.43 | 0.563 | ‐ | ‐ | ‐ |
| Diabetes | 1.15 | 0.93–1.43 | 0.214 | ‐ | ‐ | ‐ |
| Chronic kidney disease | 1.72 | 1.38–2.14 | <0.001 | 1.53 | 1.21–1.93 | <0.001 |
| CRT device | 1.03 | 0.82–1.30 | 0.775 | ‐ | ‐ | ‐ |
AF, atrial fibrillation; NYHA, New York Heart Association.
Risk stratification of clinical events
Thirty‐five cardiovascular hospitalizations and 37 deaths were associated with the HeartLogic IN alert state, whereas the remaining 18 cardiovascular hospitalizations and 18 deaths occurred in the OUT of alert state. The rates of cardiovascular hospitalizations and those of the combined endpoints of cardiovascular hospitalization or death are reported in Table 3 , for the overall population and for the groups of patients stratified by device type, presence of CKD and AF on implantation. Comparisons of the event rates in the IN alert state with those in the OUT of alert state yielded incidence rate ratios ranging from 7.36 to 13.14 for cardiovascular hospitalizations and from 9.72 to 14.54 for the combined endpoint of hospitalization or death in all groups of patients (all P < 0.001). The sensitivity for detecting study endpoints and the false‐positive rate for all patients and all subgroups of patients is reported in Table S1 . After multivariate correction for CKD and AF on implantation, the occurrence of at least one HeartLogic alert was associated with the occurrence of cardiovascular hospitalizations (HR: 3.44, 95% CI: 1.22–9.76, P = 0.021) and with the combined endpoint of cardiovascular hospitalization or death (HR: 1.92, 95% CI: 1.05–3.51, P = 0.036) (Figure 2 ). Additionally, a time IN alert ≥20% was associated with cardiovascular hospitalizations (HR: 4.14, 95% CI: 2.20–7.79, P < 0.001) and with cardiovascular hospitalization or death (HR: 3.83, 95% CI: 2.45–5.98, P < 0.001).
Table 3.
Comparison of clinical event rates in the IN alert state with those in the OUT of alert state and incidence rate ratios (IRR)
| Cardiovascular hospitalizations | Cardiovascular hospitalizations or death | |||||
|---|---|---|---|---|---|---|
| IN alert state | OUT alert state | IRR (95% CI) | IN alert state | OUT alert state | IRR (95% CI) | |
| All patients (N = 568) | 0.23 (0.16–0.32) | 0.02 (0.01–0.03) | 12.98 (7.16–24.35) | 0.48 (0.37–0.60) | 0.04 (0.03–0.05) | 13.35 (8.83–20.51) |
| CRT‐D (N = 410) | 0.20 (0.13–0.30) | 0.02 (0.01–0.03) | 12.86 (6.14–28.36) | 0.46 (0.34–0.60) | 0.03 (0.02–0.05) | 14.54 (8.80–24.66) |
| ICD (N = 158) | 0.32 (0.17–0.57) | 0.02 (0.01–0.05) | 13.14 (4.56–42.65) | 0.54 (0.33–0.83) | 0.05 (0.03–0.09) | 10.95 (5.096–24.56) |
| CKD (N = 153) | 0.44 (0.28–0.65) | 0.04 (0.02–0.07) | 10.82 (5.00–25.36) | 0.82 (0.60–1.09) | 0.06 (0.03–0.09) | 14.49 (7.81–28.59) |
| Non‐CKD (N = 415) | 0.11 (0.06–0.21) | 0.01 (0.01–0.02) | 10.89 (3.99–31.17) | 0.28 (0.19–0.41) | 0.03 (0.02–0.04) | 9.72 (5.33–17.90) |
| AF (N = 100) | 0.62 (0.40–0.89) | 0.08 (0.04–0.14) | 7.36 (3.78–14.89) | 0.95 (0.69–1.29) | 0.09 (0.06–0.15) | 10.11 (5.63–18.94) |
| Non‐AF (N = 468) | 0.21 (0.13–0.31) | 0.02 (0.01–0.03) | 10.64 (5.34–21.67) | 0.28 (0.19–0.40) | 0.02 (0.01–0.04) | 12.22 (6.66–22.97) |
P < 0.001 for all patients and all subgroups of patients.
Figure 2.
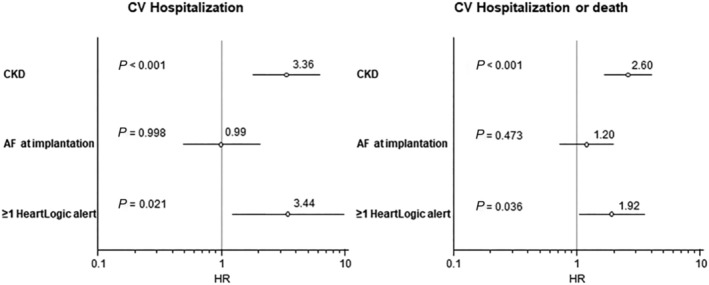
Multivariate analysis. After correction for CKD and AF on implantation, the occurrence of at least one HeartLogic alert was associated with the occurrence of the combined endpoint of cardiovascular hospitalization (HR: 3.44, 95% CI: 1.22–9.76, P = 0.021) and cardiovascular hospitalization or death (HR: 1.92, 95% CI: 1.05–3.51, P = 0.036).
Sensor data findings
The trends in the average index and sensor values surrounding the HeartLogic alert are reported in Figures S1 – S3 . On comparing the trends during clinically stable periods (average calculated 30 days prior to the alert, i.e., −60 to −30 days) we found no differences between CRT‐D and ICD patients. However, thoracic impedance was significantly lower in CKD than non‐CKD patients (Table S2). Moreover, we found a higher third sound amplitude and nocturnal heart rate, and lower first sound amplitude in AF versus non‐AF patients. These differences persisted at the time of alerts (all P < 0.05). In patients stratified by device type, CKD and AF, we measured significant changes in all contributing sensors (paired t‐test; P < 0.05) from clinically stable periods to the time of alert.
Discussion
In the present study, we evaluated the risk stratification ability of the HeartLogic algorithm in HF patients who received either CRT‐D or ICD, and we investigated the impact of common cardiovascular and non‐cardiovascular co‐morbidities. The burden of HeartLogic alerts was similar between CRT‐D and ICD patients, while patients with AF on implantation and CKD seemed more exposed to alerts. Nonetheless, the association between clinical events and the occurrence of HeartLogic alerts during follow‐up, as well as the time in the IN‐alert state, was maintained after correction for these baseline confounders, and the ability of the HeartLogic algorithm to identify periods of significantly increased risk of clinical events was confirmed both in the overall population and in patients stratified by device type, CKD and AF.
HF and CKD frequently coexist, as they share common risk factors. 14 , 15 Chronic kidney disease may worsen cardiovascular function and is a major independent determinant of increased mortality and morbidity in HF. 16 , 17 , 18 There is also a need for tools for optimizing the therapeutic management of patients with HF and concomitant CKD. Indeed, trials have shown that, although these patients are at higher risk of events, the beneficial effects of medical therapy are similar, if not greater, than in patients with normal renal function. 19 , 20 Moreover, despite differences in baseline characteristics between patients with impaired renal function and those without, no interaction between drug effects and renal function has been noted in subgroup analyses of trials. 21 , 22 , 23 , 24 Similar considerations apply to patients with AF. AF is frequent in HF, 25 , 26 and these conditions can cause or exacerbate each other through mechanisms such as structural cardiac remodelling, activation of neurohormonal systems, and rate‐related LV impairment. 27 Indeed, the development of AF in patients with chronic HF is associated with a worse prognosis, including stroke and increased mortality. 28 , 29 Hence, cases of AF coexisting with HF also require careful and specific therapeutic management. Indeed, although the relief of congestion may reduce sympathetic drive and ventricular rate and increase the probability of spontaneous return to sinus rhythm, the presence of AF may reduce or annul the prognostic benefits of beta‐blockers and render ivabradine ineffective. 30 , 31
Modern ICD diagnostic algorithms continuously measure clinical variables and have been designed to provide early warning of changes in HF status and to allow prompt intervention to prevent disease progression. Specifically, multiparameter algorithms combine data from multiple sensors which record parameters (heart rate and respiratory rate, rapid shallow breathing index, third and first heart sounds, thoracic impedance and activity) that are objective measurements of the underlying pathophysiology associated with signs and symptoms of worsening HF. 32 , 33 , 34 , 35 , 36 This system displayed high sensitivity and long warning times both in the validation study 11 and in subsequent clinical experiences. 37 , 38 , 39 The IN or OUT of alert state defined by the algorithm has also proved able to identify periods when patients are at significantly increased risk of worsening HF, 12 , 40 potentially allowing resources to be better targeted to this vulnerable patient population. In the present analysis, we confirmed the risk stratification ability of the HeartLogic index, as well as high values of sensitivity for detecting major clinical events and low false‐positive rates, in subgroups of patients not studied in previous investigations. Indeed, non‐CRT ICD patients were not included in the seminal MultiSENSE study. 11 The follow‐up of these patients may differ from that of CRT patients, as they do not benefit from the well‐known post‐implantation improvements induced by CRT in terms of cardiac function and functional capacity. Moreover, the potentially different triggering mechanisms of worsening HF episodes, which in CRT patients are frequently linked to the loss of biventricular pacing owing to suboptimal device programming, premature ventricular complexes or uncontrolled ventricular rate during AF, 41 , 42 might result in a different performance of the diagnostic algorithm and its contributing sensors. Indeed, the association between biventricular pacing percent and multiple sensor changes in patients with HeartLogic‐enabled CRT devices has recently been shown. 43 Similarly, a different performance of the diagnostic algorithm could not be excluded in patients with AF, because ventricular rate is one of the contributing parameters of the combined index, and the irregular heart rate could have impacted the accelerometer‐based assessment of first and third heart sounds. Moreover, patients with CKD might experience changes in fluid status that affect the assessment of the thoracic impedance index component. 44 In our analysis of the average sensor values during clinically stable periods, we noted a higher nocturnal heart rate in AF patients, together with higher third sound and lower first sound amplitude, that is, possible signs of chronically reduced ventricular efficiency. Moreover, in CKD patients, we found lower baseline thoracic impedance, that is, chronic fluid overload. These differences, which also persisted at the time of alerts, demonstrated the sensitivity of the ICD sensors to the worse chronic clinical conditions of patients affected by co‐morbidities, but did not impact the detection ability of the algorithm, because all ICD sensors equally contributed to the HeartLogic alerts in all patient subgroups. The differences in sensor values might suggest the possibility of adapting the weight of each individual component of the combined index according to the patient's clinical profile, in order to improve the detection accuracy. However, given the good performance of the existing algorithm demonstrated in all subgroups, increasing the complexity of the system and requiring information to be entered manually do not seem necessary. The system allows customization of the index threshold which, as demonstrated in the MultiSENSE study, 11 allows to improve sensitivity or minimize unexplained alerts. In this study, the centres did not adjust the threshold which was set to the nominal value in all patients. However, Gardner et al. demonstrated high risk stratification performance for the entire range of configurable thresholds. 12 In conclusion, we confirmed that, in patients with HeartLogic‐enabled ICD or CRT‐D, the risk of clinical events is significantly increased during IN alert periods, regardless of the type of device and the presence of cardiovascular and non‐cardiovascular co‐morbidities. As these conditions are known to affect disease severity and prognosis, AF and CKD patients could be those who benefit most from the addition of advanced tools for remote disease management in the diagnostic and prognostic armamentarium, which currently includes clinical assessment, non‐invasive and invasive testing, and natriuretic peptide assessment. Moreover, although N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) assessment is recommended in HF for prognostication, 45 AF and CKD are among the causes that might reduce its diagnostic accuracy. 1 In patients with these conditions, the HeartLogic algorithm might be of help. Indeed, the alert status has been shown to augment the ability of baseline NT‐proBNP to stratify HF risk, 12 and recently the IN alert status has proved to be associated with higher NT‐proBNP values. 39 These results are reassuring from the point of view of the diagnostic accuracy of the algorithm. However, a preventive treatment strategy in response to alerts must be implemented to obtain a better outcome for monitored HF patients. Some studies have already suggested the potential value of a timely therapeutic intervention in response to alerts. 40 , 46 However, additional studies are required in order to test the efficacy of specific interventions.
Limitations
The main limitation of this study is its observational design. Moreover, physicians were not blinded to the HeartLogic index, and no predetermined actions were prescribed in response to alerts; this may have introduced a bias into our analysis of the risk stratification ability of the algorithm. In addition, the baseline assessment of the renal function was based on local measurements by the investigators, with no standardization of assay methods.
Conclusions
In the present study, the burden of HeartLogic alerts was similar between CRT‐D and ICD patients, while patients with AF and CKD seemed more exposed to alerts. Nonetheless, the ability of the HeartLogic algorithm to identify patients during periods of significantly increased risk of clinical events was confirmed, regardless of the type of device and the presence of AF or CKD.
Conflict of interest statement
M. Campari and S. Valsecchi are employees of Boston Scientific, Inc. No other conflicts of interest exist.
Supporting information
Figure S1. Average HeartLogic index and sensor values surrounding the HeartLogic alert. Patients are stratified according to device type (CRT‐D versus ICD).
Figure S2. Average HeartLogic index and sensor values surrounding the HeartLogic alert. Patients are stratified according to the presence of chronic kidney disease at baseline.
Figure S3. Average HeartLogic index and sensor values surrounding the HeartLogic alert. Patients are stratified according to the presence of atrial fibrillation on implantation.
Table S1. Sensitivity for detecting study endpoints and false‐positive rate for all patients and all subgroups of patients.
Table S2. Matched sensor data during Baseline (−60 to −30 days) and Alert (0 to 6 days), stratified by device type, chronic kidney disease and atrial fibrillation on implantation.
Data S1. Supporting Information.
Santobuono, V. E. , Favale, S. , D'Onofrio, A. , Manzo, M. , Calò, L. , Bertini, M. , Savarese, G. , Santini, L. , Dello Russo, A. , Lavalle, C. , Viscusi, M. , Amellone, C. , Calvanese, R. , Arena, G. , Pangallo, A. , Rapacciuolo, A. , Porcelli, D. , Campari, M. , Valsecchi, S. , and Guaricci, A. I. (2023) Performance of a multisensor implantable defibrillator algorithm for heart failure monitoring related to co‐morbidities. ESC Heart Failure, 10: 2469–2478. 10.1002/ehf2.14416.
Clinical Trial Registration: URL: http://clinicaltrials.gov/Identifier: NCT02275637.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . ESC scientific document group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Goldenberg I, Huang DT, Nielsen JC. The role of implantable cardioverter‐defibrillators and sudden cardiac death prevention: indications, device selection, and outcome. Eur Heart J. 2020; 41: 2003–2011. [DOI] [PubMed] [Google Scholar]
- 3. Guaricci AI, Masci PG, Muscogiuri G, Guglielmo M, Baggiano A, Fusini L, Lorenzoni V, Martini C, Andreini D, Pavon AG, Aquaro GD, Barison A, Todiere G, Rabbat MG, Tat E, Raineri C, Valentini A, Varga‐Szemes A, Schoepf UJ, de Cecco CN, Bogaert J, Dobrovie M, Symons R, Focardi M, Gismondi A, Lozano‐Torres J, Rodriguez‐Palomares JF, Lanzillo C, di Roma M, Moro C, di Giovine G, Margonato D, de Lazzari M, Perazzolo Marra M, Nese A, Casavecchia G, Gravina M, Marzo F, Carigi S, Pica S, Lombardi M, Censi S, Squeri A, Palumbo A, Gaibazzi N, Camastra G, Sbarbati S, Pedrotti P, Masi A, Carrabba N, Pradella S, Timpani M, Cicala G, Presicci C, Puglisi S, Sverzellati N, Santobuono VE, Pepi M, Schwitter J, Pontone G. CarDiac magnEtic Resonance for prophylactic Implantable‐cardioVerter defibrillAtor ThErapy in Non‐Ischaemic dilated CardioMyopathy: an international Registry. Europace. 2021; 23: 1072–1083. [DOI] [PubMed] [Google Scholar]
- 4. Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, Cowie MR. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE‐HF trial. Eur Heart J. 2011; 32: 2266–2273. [DOI] [PubMed] [Google Scholar]
- 5. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Muñoz Aguilera R, Lunati M, Yu CM, Gerritse B, Borggrefe M, for the DOT‐HF Investigators . DOT‐HF investigators. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011; 124: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 6. Hindricks G, Varma N, Kacet S, Lewalter T, Søgaard P, Guédon‐Moreau L, Proff J, Gerds TA, Anker SD, Torp‐Pedersen C. Daily remote monitoring of implantable cardioverter‐defibrillators: insights from the pooled patient‐level data from three randomized controlled trials (IN‐TIME, ECOST, TRUST). Eur Heart J. 2017; 38: 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan JM, Kitt S, Gill J, McComb JM, Ng GA, Raftery J, Roderick P, Seed A, Williams SG, Witte KK, Wright DJ, Harris S, Cowie MR. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017; 38: 2352–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whellan DJ, Ousdigian KT, Al‐Khatib SM, Pu W, Sarkar S, Porter CB, Pavri BB, O'Connor CM, PARTNERS Study Investigators . Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (program to access and review trending information and evaluate correlation to symptoms in patients with heart failure) study. J Am Coll Cardiol. 2010; 55: 1803–1810. [DOI] [PubMed] [Google Scholar]
- 9. Cowie MR, Sarkar S, Koehler J, Whellan DJ, Crossley GH, Tang WHW, Abraham WT, Sharma V, Santini M. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur Heart J. 2013; 34: 2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Søgaard P, IN‐TIME study group* . Implant‐based multiparameter telemonitoring of patients with heart failure (IN‐TIME): a randomised controlled trial. Lancet. 2014; 384: 583–590. [DOI] [PubMed] [Google Scholar]
- 11. Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, An Q, Averina V, Stolen CM, Thakur PH, Thompson JA, Wariar R, Zhang Y, Singh JP. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail. 2017; 5: 216–225. [DOI] [PubMed] [Google Scholar]
- 12. Gardner RS, Singh JP, Stancak B, Nair DG, Cao M, Schulze C, Thakur PH, An Q, Wehrenberg S, Hammill EF, Zhang Y, Boehmer JP. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events: results from the MultiSENSE study. Circ Heart Fail. 2018; 11: e004669. [DOI] [PubMed] [Google Scholar]
- 13. Gardner RS, Thakur P, Hammill EF, Nair DG, Eldadah Z, Stančák B, Ferrick K, Sriratanasathavorn C, Duray GZ, Wariar R, Zhang Y, An Q, Averina V, Boehmer JP. Multiparameter diagnostic sensor measurements during clinically stable periods and worsening heart failure in ambulatory patients. ESC Heart Fail. 2021; 8: 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015; 36: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, di Lenarda A. Prevalence and prognostic impact of non‐cardiac co‐morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community‐based study. Eur J Heart Fail. 2018; 20: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 16. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J. 2014; 35: 455–469. [DOI] [PubMed] [Google Scholar]
- 17. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016; 12: 610–623. [DOI] [PubMed] [Google Scholar]
- 18. Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019; 62: 298–302. [DOI] [PubMed] [Google Scholar]
- 19. Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, McMurray JJ. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol. 2014; 63: 853–871. [DOI] [PubMed] [Google Scholar]
- 20. House AA. Management of Heart Failure in advancing CKD: Core curriculum 2018. Am J Kidney Dis. 2018; 72: 284–295. [DOI] [PubMed] [Google Scholar]
- 21. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐la Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. EMPEROR‐reduced trial investigators. Cardiovascular and renal outcomes with Empagliflozin in heart failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 22. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O’Connor CM. VICTORIA study group. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020; 382: 1883–1893. [DOI] [PubMed] [Google Scholar]
- 23. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 24. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, Böhm M, Bonderman D, Cleland JGF, Corbalan R, Crespo‐Leiro MG, Dahlström U, Echeverria Correa LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Lund M, Macdonald P, Mareev V, Momomura SI, O'Meara E, Parkhomenko A, Ponikowski P, Ramires FJA, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Abbasi SA, Varin C, Malik FI, Kurtz CE, GALACTIC‐HF Investigators . Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: GALACTIC‐HF baseline characteristics and comparison with contemporary clinical trials. Eur J Heart Fail. 2020; 22: 2160–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016; 13: 131–147. [DOI] [PubMed] [Google Scholar]
- 26. Carlisle MA, Fudim M, DeVore AD, Piccini JP. Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail. 2019; 7: 447–456. [DOI] [PubMed] [Google Scholar]
- 27. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016; 68: 2217–2228. [DOI] [PubMed] [Google Scholar]
- 28. Swedberg K, Olsson LG, Charlesworth A, Cleland J, Hanrath P, Komajda M, Metra M, Torp‐Pedersen C, Poole‐Wilson P. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long‐term treatment with beta‐blockers: results from COMET. Eur Heart J. 2005; 26: 1303–1308. [DOI] [PubMed] [Google Scholar]
- 29. Mogensen UM, Jhund PS, Abraham WT, Desai AS, Dickstein K, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Køber L, McMurray JJV. PARADIGM‐HF and ATMOSPHERE investigators and committees. Type of atrial fibrillation and outcomes in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2017; 70: 2490–2500. [DOI] [PubMed] [Google Scholar]
- 30. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson Å, Wikstrand J, Kotecha D, Beta‐blockers in Heart Failure Collaborative Group . Beta‐blockers for heart failure with reduced, mid‐range, and preserved ejection fraction: an individual patient‐level analysis of double‐blind randomized trials. Eur Heart J. 2018; 39: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, von Lueder T, Wedel H, Rosano G, Shibata MC, Rigby A, Flather MD, Beta‐Blockers in Heart Failure Collaborative Group . Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual‐patient data meta‐analysis. Lancet. 2014; 384: 2235–2243. [DOI] [PubMed] [Google Scholar]
- 32. Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M. Heart rate working group. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007; 50: 823–830. [DOI] [PubMed] [Google Scholar]
- 33. Forleo GB, Santini L, Campoli M, Malavasi M, Scaccia A, Menichelli M, Riva U, Lamberti F, Carreras G, Orazi S, Ribatti V, di Biase L, Lovecchio M, Natale A, Valsecchi S, Romeo F. Long‐term monitoring of respiratory rate in patients with heart failure: the multiparametric heart failure evaluation in implantable cardioverter‐defibrillator patients (MULTITUDE‐HF) study. J Interv Card Electrophysiol. 2015; 43: 135–144. [DOI] [PubMed] [Google Scholar]
- 34. Calò L, Capucci A, Santini L, Pecora D, Favale S, Petracci B, Molon G, Bianchi V, Cipolletta L, de Ruvo E, Ammirati F, la Greca C, Campari M, Valsecchi S, D’Onofrio A. ICD‐measured heart sounds and their correlation with echocardiographic indexes of systolic and diastolic function. J Interv Card Electrophysiol. 2020; 58: 95–101. [DOI] [PubMed] [Google Scholar]
- 35. Cao M, Gardner RS, Hariharan R, Nair DG, Schulze C, An Q, Thakur PH, Kwan B, Zhang Y, Boehmer JP. Ambulatory monitoring of heart sounds via an implanted device is superior to auscultation for prediction of heart failure events. J Card Fail. 2020; 26: 151–159. [DOI] [PubMed] [Google Scholar]
- 36. Yu CM, Wang L, Chau E, Chan RHW, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005; 112: 841–848. [DOI] [PubMed] [Google Scholar]
- 37. Capucci A, Santini L, Favale S, Pecora D, Petracci B, Calò L, Molon G, Cipolletta L, Bianchi V, Schirripa V, Santobuono VE, la Greca C, Campari M, Valsecchi S, Ammirati F, D'Onofrio A. Preliminary experience with the multisensor HeartLogic algorithm for heart failure monitoring: a retrospective case series report. ESC Heart Fail. 2019; 6: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santini L, D'Onofrio A, Dello Russo A, Calò L, Pecora D, Favale S, Petracci B, Molon G, Bianchi V, De Ruvo E, Ammirati F, La Greca C, Campari M, Valsecchi S, Capucci A. Prospective evaluation of the multisensorHeartLogicalgorithm for heart failure monitoring. Clin Cardiol. 2020; 43: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Juan Bagudá J, Gavira Gómez JJ, Pachón Iglesias M, Cózar León R, Escolar Pérez V, González Fernández Ó, Rivas Gándara N, Goirigolzarri Artaza J, Díaz Molina B, Macías Gallego A, Martínez Mateo V, Martínez Martínez JG, Marrero Negrín N, Alonso Salinas GL, González Torres L, Delgado Jiménez JF, Sánchez‐Aguilera P, Díaz Infante E, Arcocha Torres MF, Peña Conde L, Méndez Fernández AB, Pérez Castellano N, Rubín López JM, Madrazo Delgado I, Fernández‐Anguita MJ, Ramos Ruiz P, Medina Moreno O, Cordero Pereda D, de Diego Rus C, Arribas Ynsaurriaga F, García Bolao I, Salguero Bodes R, RE‐HEART Registry group . Remote heart failure management using the HeartLogic algorithm. RE‐HEART Registry Rev Esp Cardiol (Engl Ed). 2021; S1885‐5857: 340–346. [DOI] [PubMed] [Google Scholar]
- 40. Calò L, Bianchi V, Ferraioli D, Santini L, dello Russo A, Carriere C, Santobuono VE, Andreoli C, la Greca C, Arena G, Talarico A, Pisanò E, Santoro A, Giammaria M, Ziacchi M, Viscusi M, de Ruvo E, Campari M, Valsecchi S, D’Onofrio A, Minati M, Tota C, Martino A, Tavoletta V, Manzo M, Ammirati F, Mahfouz K, Colaiaco C, Guerra F, Zorzin Fantasia A, Amato V, Savarese G, Pellegrini D, Pimpinicchio L, Pecora D, Bartoli C, Borrello VM, Ratti M, de Rosa F, Quirino F, Tomaselli C, Marino E, Baiocchi C, de Vivo O, Baccani B, Amellone C, Lucciola MT, Angeletti A, Frisoni J, Brignoli M, Costa A, Pangallo A, Benedetto F, Pepi P, Nicolis D, Petracci B, Giubilato G, Carbonardi L, Porcelli D, Romani B, Zuccaro LM. Multiparametric implantable cardioverter‐defibrillator algorithm for heart failure risk stratification and management: An analysis in clinical practice. Circ Heart Fail. 2021; 14: e008134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruwald AC, Kutyifa V, Ruwald MH, Solomon S, Daubert JP, Jons C, Brenyo A, McNitt S, Do D, Tanabe K, al‐Ahmad A, Wang P, Moss AJ, Zareba W. The association between biventricular pacing and cardiac resynchronization therapy‐defibrillator efficacy when compared with implantable cardioverter defibrillator on outcomes and reverse remodelling. Eur Heart J. 2015; 36: 440–448. [DOI] [PubMed] [Google Scholar]
- 42. Boriani G, Gasparini M, Landolina M, Lunati M, Proclemer A, Lonardi G, Iacopino S, Rahue W, Biffi M, DiStefano P, Grammatico A, Santini M, on behalf of the ClinicalService cardiac centres . Incidence and clinical relevance of uncontrolled ventricular rate during atrial fibrillation in heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail. 2011; 13: 868–876. [DOI] [PubMed] [Google Scholar]
- 43. Cao M, Stolen CM, Ahmed R, Schloss EJ, Lobban JH, Kwan B, Varma N, Boehmer JP. Small decreases in biventricular pacing percentages are associated with multiple metrics of worsening heart failure as measured from a cardiac resynchronization therapy defibrillator. Int J Cardiol. 2021; 335: 73–79. [DOI] [PubMed] [Google Scholar]
- 44. Schoutteten MK, Vranken J, Lee S, Smeets CJP, de Cannière H, van Hoof C, Peeters J, Groenendaal W, Vandervoort PM. Towards personalized fluid monitoring in haemodialysis patients: thoracic bioimpedance signal shows strong correlation with fluid changes, a cohort study. BMC Nephrol. 2020; 21: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh T. N‐terminal pro‐brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003; 24: 1735–1743. [DOI] [PubMed] [Google Scholar]
- 46. Guerra F, D'Onofrio A, De Ruvo E, Manzo M, Santini L, Giubilato G, La Greca C, Petracci B, Stronati G, Bianchi V, Martino A, Franculli F, Compagnucci P, Campari M, Valsecchi S, Dello Russo A. Decongestive treatment adjustments in heart failure patients remotely monitored with a multiparametric implantable defibrillators algorithm. Clin Cardiol. 2022; 45: 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Average HeartLogic index and sensor values surrounding the HeartLogic alert. Patients are stratified according to device type (CRT‐D versus ICD).
Figure S2. Average HeartLogic index and sensor values surrounding the HeartLogic alert. Patients are stratified according to the presence of chronic kidney disease at baseline.
Figure S3. Average HeartLogic index and sensor values surrounding the HeartLogic alert. Patients are stratified according to the presence of atrial fibrillation on implantation.
Table S1. Sensitivity for detecting study endpoints and false‐positive rate for all patients and all subgroups of patients.
Table S2. Matched sensor data during Baseline (−60 to −30 days) and Alert (0 to 6 days), stratified by device type, chronic kidney disease and atrial fibrillation on implantation.
Data S1. Supporting Information.


