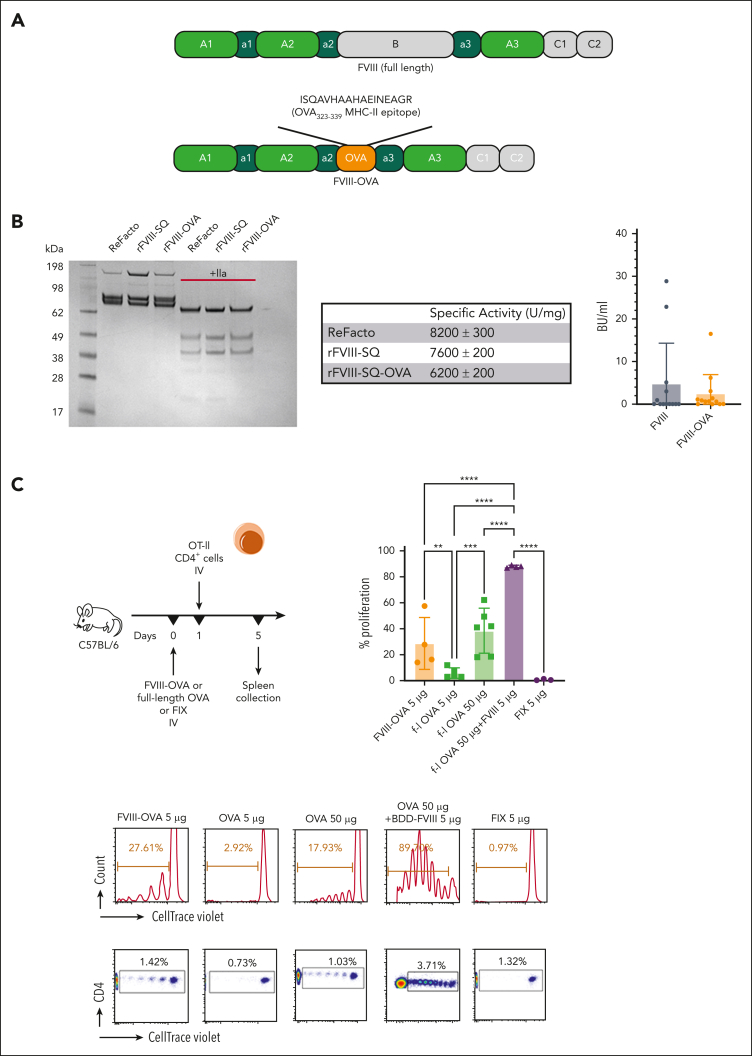
Figure 3.
Systemically administered FVIII-OVA induces more robust in vivo proliferation of CD4+ T cells than OVA. (A) Schematic diagram of FVIII-OVA (FOVA) in comparison with WT FVIII. In FOVA, the B domain was replaced with the immunodominant MHC-II epitope of chicken OVA. (B) Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of and inhibitor formation in response to FOVA (mean ± SD). Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of 2 μg rFVIII-SQ and rFVIII-SQ-OVA in the presence or absence of 100 nM thrombin. The gel was stained with Coomassie blue. A comparison with a commercially available rFVIII, ReFacto, is shown. Specific activity was determined by partial thromboplastin time one-stage clotting assay measurement relative to protein concentration. Data are shown as mean ± standard error measurements from duplicate experiments. For inhibitor assay, C57BL/6 HA mice received 4 weekly IV injections of 1.5 IU (300 ng) FOVA or B domain-deleted (BDD)-FVIII. Inhibitor titers were measured 1 week after the last injection. (C) WT C57BL/6 mice (n = 3-6) received 5 μg FOVA, full-length OVA (5 μg or 50 μg), BDD-FVIII (5 μg) + full-length OVA (50 μg), or FIX (5 μg) IV. One day later, CD4+ T cells were magnetically sorted from OT-II mice, labeled with CTV, and adoptively transferred to the experimental C57BL/6 mice. Four days later the spleens were collected for flow cytometry analysis of proliferation. The plots show a summary of percentage proliferation (mean ± SD), representative proliferation histograms, and respective dot plots.