Figure 2.
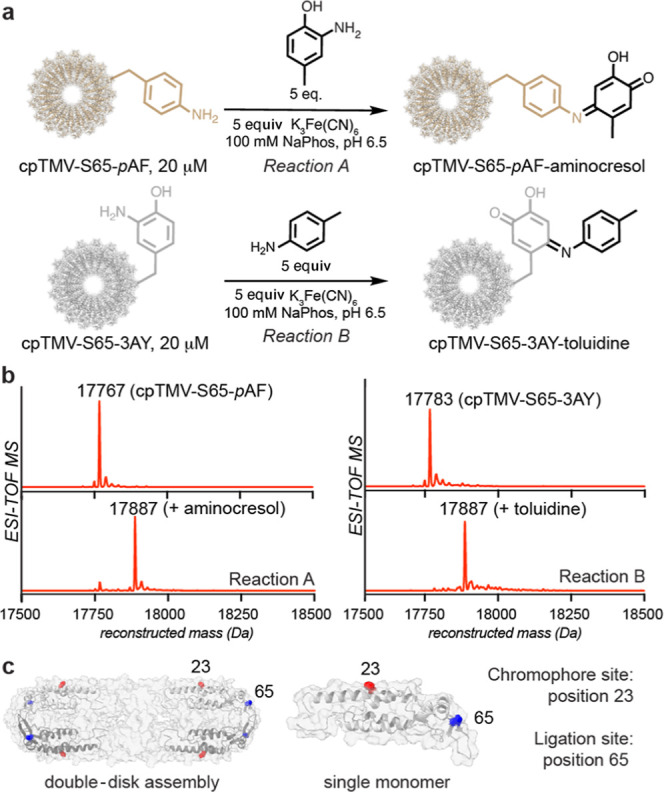
Chemical confirmations for accessibility and modification of ncAA-containing amino acids through small-molecule couplings. (a) Conditions are shown for K3Fe(CN)6-mediated oxidative coupling to both cpTMV-S65-pAF (reaction A) and cpTMV-S65-3AY (reaction B). (b) Reconstructed ESI-TOF mass spectra indicated high conversion of each ncAA-containing cpTMV monomer to the expected oxidative coupling product (expected MW: 17,887 Da). (c) A cutaway view is provided, showing cpTMV monomers on opposite sides of the disk in gray, sites for protein–protein conjugation in blue, and sites for pigment attachment in red. A close-up view of a single monomer of the individual double-disk assembly is also shown.
