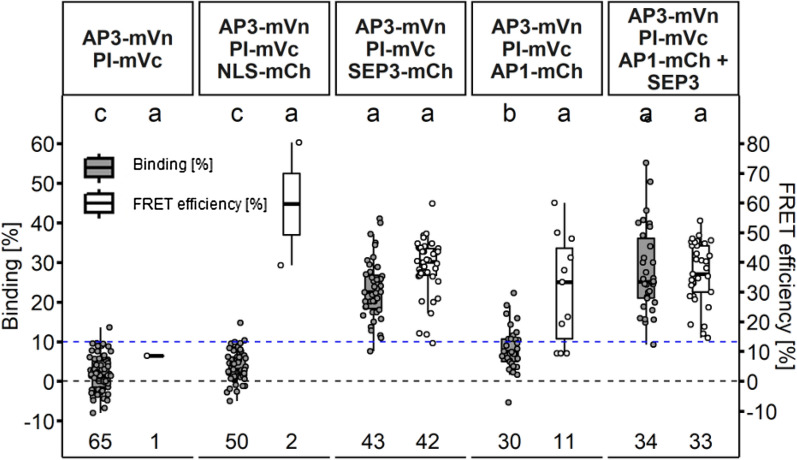
Fig. 11.
Larger complex formation between AP1, AP3, PI and SEP3 proteins in N. benthamiana. Higher order complex formation of MADS-box proteins was analysed by a combination of BiFC with FRET-FLIM. Complemented mV by the n-terminal and c-terminal part of mV tagged to AP3 and PI respectively served as the donor in FRET-FLIM experiments. All MADS-domain protein fused to FP were expressed via the UBQ10 promoter. Untagged SEP3 was expressed from the same T-DNA as AP1-mCh using the XVE < < oLexA-35S estradiol inducible system. Images were acquired three days after infiltration and SEP3 was induced one day before imaging. BINDING [%] (grey) and FRET efficiencies [%] (white) for AP3-mVn PI-mVc, AP3-mVn PI-mVc NLS-mCh, AP3-mVn PI-mVc SEP3-mCh, AP3-mVn PI-mVc AP1-mCh and AP3-mVn PI-mVc AP1-mCh + SEP3. Analysis was done as described in Fig. 5. SEP3 together with AP3/PI displayed increased BINDING (23.21% ± 7.56) with a FRET efficiency of 38.57% ± 9.50. Affinity of AP1 for AP3/PI was low (8.40% ± 5.66 BINDING), but strongly increased in presence of SEP3 (28.80% ± 12.36 BINDING). Statistical groups were assigned after multiple comparison with Kruskal–Wallis and a Post hoc test using the criterium Fisher’s least significant difference (alpha parameter is 0.05) (Dashed blue line marks the BINDING cut-off of 10%; number of repetitions are indicated below BINDING values and number of images with BINDING above 10% are indicated below the FRET efficiency values in the bottom of the plot)