Highlights
-
•
The analysis demonstrated the utility of oncotype DX® RS in a population of older breast cancer patients.
-
•
After testing with oncotype DX®, older patients received less chemotherapy despite having a higher proportion of high-risk recurrence scores.
-
•
Oncotype DX® should be prescribed after consultation with older patients to assure benefits.
Keywords: Early breast cancer, 21-gene score, Older patients, Estrogen receptor positive, Chemotherapy, Endocrine therapy
Abstract
Background
In early luminal breast cancer, the Oncotype DX® Recurrence Score (RS) prognostic and predictive value with regards to chemotherapy (CHT) application benefit has been broadly validated. In older patients its value has not been deeply addressed. This study aimed to evaluate the benefits of RS testing and to look at differences in treatment allocation for these patients when compared with younger ones.
Methods
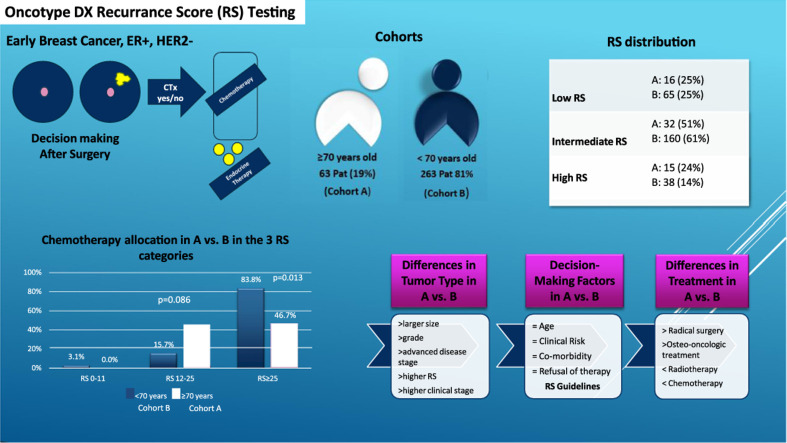
We included data from consecutive patients with early luminal HER2-negative breast cancer, treated between 2010 and 2022 at the University Hospital Basel and Cantonal Hospital Baselland, Switzerland. The older cohort included 63 (19%) patients aged ≥70, and the younger cohort 263 (81%) patients aged <70.
Results
Older breast cancer patients had more co-morbidities (N = 36, 57% vs. N = 92, 35%, p = 0.002) and a higher clinical risk status (N = 49, 78% vs. N = 155, 59%; p = 0.01) when compared to younger patients. Histopathologic characteristics were significantly different between the two cohorts. Although older patients had a higher clinical risk status (78% vs. 59%) (p = 0.01), most of them (74%) received no CHT. Specifically, adjuvant CHT was administered less frequently in older than in younger patients (13% vs. 22%; p = 0.01). Moreover, older patients were less likely to complete CHT (>4 cycles: 78% vs. 97%).
Conclusion
Breast cancer patients aged ≥70 have higher clinical risk status, more co-morbidities, higher clinical stage (driven by larger tumor size), and more often RS ≥26. However, they receive fewer adjuvant RT and CHT than those aged <70. RS maintains its independent prognostic value in older patients. However, assessing the predictive value of additional CHT benefit remains challenging due to significant differences in CHT administration. Although therapy decision-making in older patients with breast cancer still follows RS-based guidelines, clinical practice indicates an individualized treatment approach.
Graphical abstract
Introduction
To improve decision-making regarding the applicability of adjuvant chemotherapy (CHT), genetic signatures have been introduced to clinical practice (Sparano et al., 2020). Among them, tests such as Oncotype DX, MammaPrint, EndoPredict, PAM50, help identify tumors that may benefit from additional CHT (Cardoso et al., 2016; F. Fitzal et al., 2015). Over the past decade, this has been a pivoting point in personalized cancer treatment, especially in low-risk breast cancer, as several of these signatures have been incorporated in clinical practice guidelines and have had a significant impact on cost-effective adjuvant therapy decision-making. The Oncotype DX Recurrence Score (RS) is commonly used in selecting patients who may benefit from CHT and is currently the test of choice at the Basel University Hospital and Cantonal Hospital Baselland.
In older patients with breast cancer, the addition of adjuvant CHT can be problematic despite its benefits in node-positive disease. Particularly, co-morbidities and frailty must be addressed to optimize treatment decisions. The benefits of RS testing in an older population have not been thoroughly addressed. Moreover, results from the TAILORx and RxPonder trials showed no benefit for postmenopausal patients or those aged >50 years [1]. Despite this, the Oncotype DX RS remains the most used prognostic tool, even in older patients with breast cancer.
The majority (80%) of newly diagnosed patients with breast cancer each year, are >50 years old, of which 35% are >70 years old. Although major improvements have been achieved in terms of treatment and reduction in the overall mortality rate in the breast cancer population, older patients seem to have not benefited from all these advancements [2]. Moreover, standard treatment is often overlooked in the elderly, due to shortened life expectancy, advanced stage at presentation, organ dysfunction, multi-morbidity, and frailty [3].
In a SEER study, CHT in older patients with high RS did not lead to improved survival when compared to younger patients. Older patients tended to receive CHT less often (50%) than younger patients (70%). However, no additional benefit of CHT was documented in the older population [4]. On the contrary, age is an independent negative prognostic factor and therefore, some physicians recommend adding CHT for older patients based on clinical risk and life expectancy > 5 years [5]. These findings are convergent with those of other SEER-based studies, which showed that age and RS are independent prognostic factors [6].
The purpose of this retrospective study was to evaluate the benefits of RS testing in breast cancer patients >70 years of age and assess the difference in treatment allocation and impact on disease free survival (DFS) and overall survival (OS) when compared to younger patients. This study makes a novel contribution to the literature by assessing utility and frequency of RS testing in elderly breast cancer patients.
Material and methods
Patient selection and data analysis
This was a retrospective analysis of data available from patients with early luminal HER2-negative breast cancer treated at Basel University Hospital and Cantonal Hospital Baselland between 2010 and 2022. Patients’ data were anonymized. The Study was approved by the local ethics committee (Ethics Committee Nord-West-Schweiz, www.eknz.ch).
The inclusion criteria were patients who were 1) >18 years old, 2) diagnosed with breast cancer between 2010 and 2022, 3) had a valid RS result on file and 4) underwent at least one therapeutic option (endocrine therapy, radiotherapy [RT], and/or CHT). All clinical and pathological data, including age, tumor stage, co-morbidities, family history, risk factors (BMI, history of hormonal therapy, parity, and previous cancer), tumor grading, size, location, nodal status, estrogen receptor (ER), progesterone receptor (PR), Ki-67 expression and histologic type were collected. Therapy profiling (type of surgery, type of CHT - including number of cycles, type of RT, including dosage, type of endocrine therapy and osteo-oncologic therapy) and any associated side effects, treatment interruption, the tumor board's decision, therapy administration, and RS-based treatment options were also registered.
Definition of clinical risk
Low clinical risk was defined as a tumor of ≤1 cm and G3, ≤ 2 cm and G2, or ≤ 3 cm and G1. High clinical risk was defined as the presence of all other tumor types. Multi-morbidity was defined as ≥3 chronic illnesses, including cardiac disease, diabetes, COPD and asthma. At the tumor board meetings, therapeutic decisions were made in a multidisciplinary manner. In most cases, RS was known and presented with other clinical and pathological characteristics.
All data were collected from the hospital databases (ISMED, CATO, Tumor Center database, Breast Cancer Center, Gynecology Department, and Medical Oncology Department), coded by patient number and stored in a Microsoft Excel spreadsheet. Selected variables were denoted as numerical or categorical (yes/no) type values to facilitate statistical processing. The level of statistical significance was set at p<0.05. They were further grouped into categories based on demographics, co-morbidities, tumor characteristics, RS, therapy regimen, intensity and side effects, convergence/divergence of Tumor Board decision with the given RS result and outcome.
Endpoints
The primary endpoint was to determine whether clinical practice still follows the recommended RS-based guidelines in the case of older breast cancer patients, or if decisions are driven by multi-morbidity or clinical risk assessment.
As secondary endpoints, we evaluated differences in clinical risk, tumor characteristics, comorbidities, type and intensity of therapy received (surgery, CHT, endocrine, and osteo-oncologic), and main factors leading to therapy decision-making between older and younger patient groups.
Results
Baseline characteristics
A total of 326 patients with early luminal, HER2-negative, breast cancer who underwent RS testing and had an official recorded score were selected. Of these, 319 were female (97.9%). Men comprised 3% (2 patients) of the older group and 2% (5 patients) of the younger cohort (p = 0.61).
The median age at diagnostic was 59 years. Sixty-three patients (19%) were ≥70 years old at moment of diagnostic (older group), with a median age of 74 years (range 70–85 years), while the remaining 263 patients (81%) were <70 years old (younger group), with a median age of 54 (range 29–69 years) (p<0.001).
Older patients had at least one relevant co-morbidity (N = 36, 57%) when compared to younger patients (N = 92, 35%) (p = 0.002). Multimorbidity (>3 other diseases) was identified in 38% of older patients vs only 4% younger patients. Older patients appear to have less frequent germline mutations however, genetic testing was rarely performed in this group compared to the younger group of patients (13% vs 33%). Patient demographics are summarized in Table 1.
Table 1.
Patient Demographics.
| Total population | Younger group | Older group | p-value | ||
|---|---|---|---|---|---|
| Number | 326 | 263 (80.67%) | 63 (19.32%) | ||
| Age (median) | 59.0 [29.0;85.0] | 54.0 [29.0;69.0] | 73.0 [70.0;85.0] | <0.001 | |
| Women |
319 (97.85%) | 258 (98.1%) | 61 (98.8%) | 0.624 | |
| Men | 7 (2.15%) | 5 (1.90%) | 2 (3.17%) | ||
| Menopause | <0.001 | ||||
| No | 82 (25.7%) | 82 (31.8%) | 0 (0.00%) | ||
| Yes | 216 (67.7%) | 155 (60.1%) | 61 (100%) | ||
| Peri | 21 (6.58%) | 21 (8.14%) | 0 (0.00%) | ||
| Relevant co-morbidities | 0.002 | ||||
| 128(39.3%) | 92 (35%) | 36 (57.1%) | |||
| CVD | 104(100%) | 71 (100%) | 33 (100%) | ||
| History of cancer* | 24(100%) | 18 (100%) | 6 (100%) | ||
| BC history | 27 (100%) | 16 (100%) | 11 (100%) | ||
| BMI | 0.147 | ||||
| <18.5 | 10 (3.17%) | 9 (3.54%) | 1 (1.64%) | ||
| 18.5–24.9 | 155 (49.2%) | 132 (52.0%) | 23 (37.7%) | ||
| 25–29.9 | 96 (30.5%) | 69 (27.2%) | 27 (44.3%) | ||
| 30–34.9 | 37(11.7%) | 29 (11.4%) | 8 (13.1%) | ||
| 35–39.9 | 12 (3.81%) | 10 (3.94%) | 2 (3.28%) | ||
| >40 | 5 (1.59%) | 5 (1.97%) | 0 (0.00%) | ||
| Positive family history* | 0.399 | ||||
| No | 236 (76.9%) | 188 (75.2%) | 48 (84.2%) | ||
| Yes | 69 (22.5%) | 60 (24%) | 9 (15.8%) | ||
| Germline mutation | 0.023 | ||||
| No | 74 (24.7%) | 68 (28%) | 6 (10.5%) | ||
| BRCA1/2 | 8 (2.67%) | 7 (2.88%) | 1 (1.75%) | ||
| Other | 11 (3.67%) | 10 (4.12%) | 1 (1.75%) | ||
| Not tested | 206 (68.7%) | 157 (64.6%) |
|
Not breast cancer **Breast cancer in first degree relatives, CVD= cardiovascular disease, BC =breast cancer, BMI=body-mass-index.
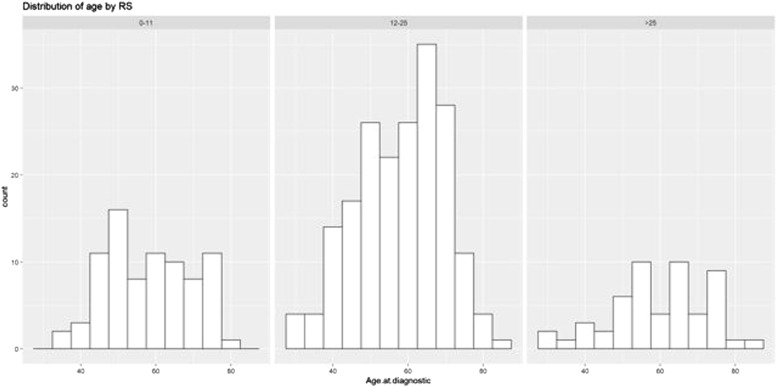
RS score was not significantly different between the two groups, with a median of 16 points in the younger cohort and 19 in the older one (p = 0.22). Fig. 1 and Table 2 show the distribution of the risk groups in the two cohorts.
Fig. 1.
RS distribution according to age groups, Figure 1. Patient distribution according to age in the three RS categories showing most patients in the intermediate score category, regardless of age.
Table 2.
Patient distribution in the RS categories.
| Low RS 0–11 | Intermediate RS 11–25 | High RS >25 | |
|---|---|---|---|
| Younger group | 65 (25%) | 160 (61%) | 38 (14%) p<0.001 |
| Older group | 16 (25%) | 32 (51%) | 15 (24%) p<0.001 |
Table 2 shows patient distribution according to age and RS result category.
Considering the RS, there was a significant difference between older and younger patients only for high scores (RS>25: 24% vs. 14%; p<0.001), while low and intermediate score were similar in both groups (RS <11: 25% vs. 25% and RS 11–25: 51% vs. 61%).
Pathology, RS distribution and stage
Primary tumor size was larger in older vs. younger patients (median 25 mm vs. 18 mm, p<0.001) accounting for tumors pT2 and above in 68% of cases vs. 42%, respectively. Similarly, older patients had more often positive clinical nodal status (29% vs. 17.5%, p = 0.05). Therefore, the overall disease stage was more advanced in older patients when compared to younger ones, with stage IIB or above in 37% vs. 25% (p = 0.039) – see Table 3a.
Table 3.
a. Tumor Characteristics.
| Total population | Younger group | Older group | p-value | N | |
|---|---|---|---|---|---|
| Morphology | 1.000 | 77 | |||
| Multicentric | 32 (41.6%) | 25 (41.0%) | 7 (43.8%) | ||
| Multifocal | 45 (58.4%) | 36 (59.0%) | 9 (56.2%) | ||
| Tumor size | 20.0 [3.50;130] | 18.0 [3.50;130] | 25.0 [6.00;120] | <0.001 | 325 |
| Nodal status | 0.400 | 315 | |||
| Negative | 189 (58.7%) | 149 (57.3%) | 40 (64.5%) | ||
| Positive | 133 (41.3%) | 111 (42.7%) | 22 (35.5%) | ||
| cT stage | 0.274 | 148 | |||
| 0 | 1 (0.68%) | 1 (0.83%) | 0 (0.00%) | ||
| 1 | 70 (47.3%) | 62 (50.4%) | 8 (32%) | ||
| 2 | 69 (46.6%) | 53 (43.1%) | 16 (64%) | ||
| 3 | 8 (5.41%) | 7 (5.69%) | 1 (4.00%) | ||
| cN stage | 0.057 | 144 | |||
| 0 | 116 (80.6%) | 99 (82.5%) | 17 (70.8%) | ||
| 1 | 25 (17.4%) | 20 (16.7%) | 5 (20.8%) | ||
| 1(i) | 1 (0.69%) | 0 (0.00%) | 1 (4.17%) | ||
| 2 | 1 (0.69%) | 0 (0.00%) | 1 (4.17%) | ||
| 2b | 1 (0.69%) | 1 (0.83%) | 0 (0.00%) | ||
| Number of cN | 0.028 | 120 | |||
| 0 | 92 (76.7%) | 80 (79.2%) | 12 (63.2%) | ||
| 1 | 26 (21.7%) | 21 (20.8%) | 5 4 (26.3%) | ||
| 2 | 2 (1.67%) | 0 (0.00%) | 2 (10.5%) | ||
| Staging | 0.039 | 326 | |||
| IA | 110 (33.7%) | 94 (35.7%) | 16 (25.4%) | ||
| IB | 5 (1.53%) | 5 (1.90%) | 0 (0.00%) | ||
| IIA | 121 (37.1%) | 97 (36.9%) | 24 (38.1%) | ||
| IIB | 67 (20.6%) | 51 (19.4%) | 16 (25.4%) | ||
| IIIA | 15 (4.60%) | 13 (4.94%) | 2 (3.17%) | ||
| IIIB | 2 (0.61%) | 1 (0.38%) | 1 (1.59%) | ||
| IIIC | 4 (1.23%) | 2 (0.76%) | 2 (3.17%) | ||
| IV | 2 (0.61%) | 0 (0.00%) | 2 (3.17%) | ||
| pT | <0.001 | 326 | |||
| 1 | 174 (53.4%) | 154 (58.6%) | 20 (31.7%) | ||
| 2 | 121 (37.1%) | 88 (33.5%) | 33 (52.4%) | ||
| 3 | 29 (8.90%) | 20 (7.60%) | 9 (14.3%) | ||
| 4 | 2 (0.61%) | 1 (0.38%) | 1 (1.59%) | ||
| pN | 0.132 | 324 | |||
| 0 | 188 (58%) | 148 (56.3%) | 40 (65.6%) | ||
| 1 | 127 (39.2%) | 108 (41.1%) | 19 (31.1%) | ||
| 2 | 5 (1.54%) | 5 (1.90%) | 0 (0.00%) | ||
| 3 | 4 (1.23%) | 2 (0.76%) | 2 (3.28%) | ||
| Nodal excision | 0.033 | 137 | |||
| SLN | 96 (70.1%) | 86 (74.1%) | 10 (47.6%) | ||
| SLN+Axilla | 23 (16.8%) | 16 (13.8%) | 7 (33.3%) | ||
| Axilla | 18 (13.1%) | 14 (12.1%) | 4 (19%) |
Table 3a: shows the different clinical-pathological factor in the older and younger group. ER=estrogen receptor, PR = progesterone receptor, SLN = Sentinel lymph node biopsy.
High clinical risk tumors were more prevalent in older patients (78%) than in younger patients (59%) (p = 0.01).
There were no differences in the histologic tumor type, hormonal receptor status and tumor grading – see supplemental material (Table 3b).
For both cohorts, tumors in the high RS category had less PR expression compared to intermediate and low RS categories (20% vs. 80% vs. 90% respectively, p<0.001), higher tumor grade (G3 tumors in 65% vs. 34% vs. 15%, p<0.001) and higher median Ki-67 expression (25% vs. 20% vs. 15% respectively, p<0.001). Ki-67 >20% was reported in 59%, 42% and 20%, in these RS categories (p<0.001).
Positive lymphocytic and perineural infiltration, a feature of invasion, was observed in high RS tumor cases. As such, 54% of high RS tumor samples showed signs of lymphocytic infiltration when compared to intermediate (25%) and low (22%) RS tumor samples (p = 0.01) and 25% high RS tumors showed signs of perineural infiltration when compared to intermediate (13%) and low (5%) RS (p = 0.003). High clinical risk tumors were found in the high RS group (77%) (p = 0.027).
Therapy
All patients in both groups received endocrine therapy, while CHT was recommended in 13% older patients vs. 22% younger patients (p = 0.13).
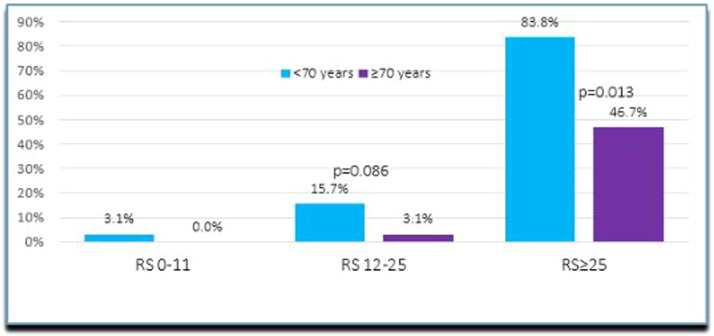
Most indications for CHT were in the intermediate (14%) and high (73%) RS groups (p<0.001). In the high RS category, younger patients (84%) received CHT more often than older patients (47%) (p = 0.013) – see Fig. 2 below. The preferred regimen (78% in the younger population and 85% in the older group) was docetaxel/cyclophosphamide for an average of 4 cycles. In the intermediate RS category, this regimen accounted for 61.5% of all prescribed drugs and in the high RS for 85% (p = 0.004).
Fig. 2.
CHT Administration according to RS in the two cohorts (<70 years of age and >70 years of age breast cancer patients).
In the older cohort no significant difference was noted in CHT for high clinical vs. low clinical risk (14% vs. 7%, p = 0.671), however more often younger patients with high clinical risk tumors received CHT (30%) vs. those with low-risk (11%) (p = 0.01) – see Table 5 in the supplement.
When treatment was considered by RS categories and without considering age, all 53 patients with high RS and only seven (4%) with intermediate RS received adjuvant CHT. None of the patient with low RS (81 patients) received additional CHT (p<0.001).
Regarding other oncological treatments, we observed a trend for a higher mastectomy rate (40% vs. 29%, p = 0.065), significantly less radiotherapy (RT) (65% vs. 81%, p = 0.009), and more osteo-oncologic treatment (61% vs. 43%, p = 0.013) in the elderly vs. younger population. Also, older women underwent reconstruction less frequently (31%) than younger ones (55%) (p = 0.014).
Older patients received less frequent RT compared to younger patients (64% vs. 81%, p = 0.01), particularly for the intermediate RS group (56% vs. 81%, p = 0.05) and had a lower total median dose (50.5 Gy (range 25–66.4 Gy) vs. 53.2 Gy (range 29–66.4 Gy), p = 0.04) – see Table 4.
Table 4.
Breast cancer management.
| Total Population | <70 years-old | >70 years-old | p value | N | |
|---|---|---|---|---|---|
| Primary Surgery | 325 (99.7%) | 262 (100%) | 62 (98.4%) | 0.193 | 326 |
| Type of breast surgery | 0.079 | ||||
| Brest conservative | 222 (68.9%) | 185 (71.2%) | 37 (59.7%) | ||
| Mastectomy | 100 (31.1%) | 75 (28.8%) | 25 (40.3%) | ||
| Type of reconstruction | 0.014 | 325 | |||
| No reconstruction | 164 (50.5%) | 121 (46.0%) | 43 (69.4%) | ||
| Reconstruction | 161 (49.5%) | 142 (54%) | 19 (30.6%) | ||
| Post-op. Complications | 40(12.4%) | 33 (12.6%) | 7 (11.3%) | 0.939 | |
| Adjuvant CHT | 66 (20.4%) | 58 (22.2%) | 8 (12.7%) | 0.131 | 324 |
| Refused | 21 (100%) | 14 (100%) | 7 (100%) | . | 21 |
| Anthracyclines + alkylating agent | 5 (7.14%) | 4 (6.67%) | 1 (10%) | 0.105 | 70 |
| Taxanes + alkylating agent | 52 (74.3%) | 46 (76.7%) | 6 (60%) | ||
| 4 cycles | 58 (84.1%) | 51 (85%) | 7 (77.8%) | 0.095 | |
| 6 cycles | 7 (10.1%) | 7 (11.7%) | 0 (0.00%) | ||
| CHT assoc. complications | 11 (16.4%) | 10 (16.9%) | 1 (12.5%) | 1.000 | 67 |
| RT | 0.009 | 325 | |||
| Refusal | 20 (100%) | 15 (100%) | 5 (100%) | . | |
| RT Adjuvant | 253 (77.8%) | 213 (81.3%) | 40 (63.5%) | 0.004 | 325 |
| Total Dose (Gy) | 53.2 [25.0;66.4] | 53.2 [29.0;66.4] | 50.5 [25.0;66.4] | 0.041 | |
| Completed RT | 251 (77.2%) | 210 (80.2%) | 41 (65%) | 0.017 | 325 |
| Endocrine Therapy | 311 (95.4%) | 249 (94.7%) | 62 (98.4%) | 0.319 | 326 |
| Refused | 15 (100%) | 14 (100%) | 1 (100%) | 15 | |
| Adjuvant | 310 (95.1%) | 248 (94.3%) | 62 (98.4%) | 0.326 | 310 |
| Tamoxifen | 73 (23.9%) | 71 (28.9%) | 2 (3.33%) | <0.001 | |
| AI | 192 (62.7%) | 135 (54.9%) | 57 (95%) | ||
| Tamoxifen + GnRH | 3 (0.98%) | 3 (1.22%) | 0 (0.00%) | 319 | |
| AI plus GnRH | 20 (6.54%) | 20 (8.13%) | 0 (0.00%) | ||
| Sequential Tamoxifen-AI | 18 (5.88%) | 17 (6.91%) | 1 (1.67%) | ||
| Osteo-oncologic therapy | 145 (46.5%) | 108 (42.9%) | 37 (61.7%) | 0.013 | 312 |
| Denosumab | 31 (22.3%) | 20 (19.4%) | 11 (30.6%) | 0.440 | 139 |
| Bisphosphonates | 107 (77%) | 82 (79.6%) | 25 (69.4%) |
Older patients received more often osteo-oncologic (mostly bisphosphonates) treatment than younger ones (62% vs. 43%, p = 0.013), particularly in the intermediate RS category (65% vs. 43%, p = 0.043).
Tumor board recommendations, including CHT, hormonal therapy and RT, were implemented in 48 (77%) older breast cancer patients and 211 (81%) younger ones (p = 0.66).
For all patients, tumor board treatment recommendation deviated from the most recent RS guidelines in 46 (14%) cases, mainly affecting the younger population (41 cases). The distribution according to RS was 4 in the high, 38 in the intermediate and 4 in low category (p = 0.008). In the older cohort, 13 (21%) patients refused the tumor board's treatment decisions. Of these, therapy recommendation in 5 cases conflicted with RS guidelines.
Among the younger patients, 43 (16%) refused CHT. Of these, 41 also conflicted with RS based recommendation.
Factors such as patients’ refusal, physician decision, or life-threatening side effects, significantly caused this divergence in therapy decisions and RS-based recommendations (p = 0.037) – see Table 6 in the supplement.
Outcome
After median follow-up of 36 months, recurrence rate was higher in the older population vs. the younger group, although non-significant (N = 8, 13% vs. N = 16, 6.5%, p = 0.113). Recurrence rate was higher with RS ≥26 vs. RS 0–25 (13.5% vs. 5.7%, respectively; p = 0.043).
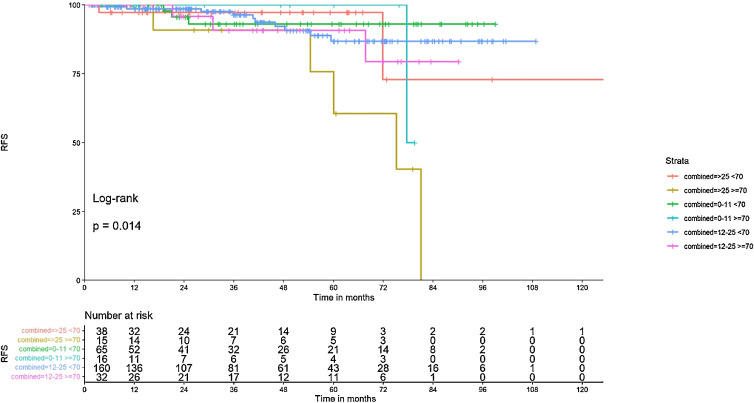
Older patients with high RS relapsed more often (5/8 cases) than younger patients (3/8 cases). Details of the outcomes are presented in Table 7 of the supplement. The age- and RS-adjusted recurrence distribution is shown in Fig. 3.
Fig. 3.
Recurrence free survival in RS and age subgroups, Figure 3 shows the correlation of recurrence/death event distribution with age and RS.
Death events were more often registered in the older group than in the younger group (3 [5%], of which 2 were breast cancer-related vs. 2 [0.7%] in the younger cohort, of which one was breast cancer-related, p = 0.05). Two deaths were recorded in the high RS group and three in the intermediate RS group (p = 0.19). When analyzing the important predictors in the OS analysis, the Cox regression coefficient showed a trend toward significance for age at diagnosis (HR 2.3, p = 0.05) and less for high or intermediate RS categories (HR 2.54, p = 0.142 and HR 1.35, p = 0.59).
Discussion
In this retrospective analysis we aimed at establishing main differences in tumor characteristics, therapy administration and outcome, in a younger vs. older cohort of Her2 negative breast cancer patients who were treated at our center over the past 12 years, in order to determine added benefit of RS testing among elderly patients.
As one of the most relevant prognostic tools available in the clinical practice, Oncotype DX RS is currently being used for therapy stratification even in older breast cancer patient populations. Modality of selection for RS testing among the elderly remains unclear, since issues such as life expectancy, comorbidity and frailty might obscure its benefit.
In our study, older patients with intermediate to high RS seemed to undergo more often RS testing in comparison to younger patients with low to intermediate RS. This may reflect a selection bias, as Oncotype DX® test seems be prescribed more often in older patients only in the presence of high clinical risk tumors, whereas is more readily available for younger breast cancer patients regardless of tumor characteristics. This stems particularly from a lack of evidence-based data regarding performance of elderly cancer patients in clinical trials, as well as a biased medical assessment of possible treatment toxicities and tolerability and an underestimation of life expectancy in this segment of population. The term therapeutic nihilism has been coined, aiming at capturing this unfortunate clinical trend [7].
Since age is considered an independent risk of tumor development, associated with a myriad of epigenetic modifications, increased age will be associated with higher statistical probability of cancer, but also with other preexisting, mostly chronic morbidities, polypharmacy, decline in cognitive function, nutritional status and social integration, ultimately leading to a biased evaluation in the clinical context and further to under-diagnosis and under-treatment [7]. The extent of this problem is to date also insufficiently investigated, since older patients are often excluded from any clinical or prospective studies. This nihilism reflects in our current work as well, through the relatively small sample size of elderly patients (63) in comparison to younger ones (263).
Regarding population characteristics in the above mentioned context, we therefore focused to determine the role of comorbidity in therapy decision-making. Older women were more often comorbid (57%) than younger ones (35%). Ten (3.8%) and 24 (38%) younger and older patients, respectively, had >3 other ongoing diseases. Comorbidity was also a significant (p = 0.047) feature of tumors with intermediate RS, with 44.3% of patients in the RS category being comorbid.
Our study showed important discrepancies in diagnostic workup between the two populations. Older patients were tested for genetic mutations less often than younger patients. The decision not to test for genetic mutations in the older population can be justified by the more aggressive tumor profile, as 78% of older patients in our study had high clinical risk tumors. Since the prevalence was similar in both cohorts, this could be a detrimental finding for the older patients, who might also have benefited from targeted therapies.
In comparison with younger patients, older patients had larger (18 vs. 25 mm) tumors and more often positive nodal status (17.5% vs. 29%), which also correlated with more aggressive molecular markers: less PR positivity, higher Ki-67%, more luminal B histologic subtype. Ki-67 distribution according to linear RS increase proves to be of particular importance, as recent guidelines suggest replacing the RS with a low/high Ki-67 proliferation marker in cases where Oncotype DX testing cannot be performed.
Ki-67 distribution was non-significant after adjusting for age (p = 0.93). In the high Ki-67 group, 23 patients (37%) were >70 years of age and 92 (35%) were <70; in the low Ki-67% group, 40 patients were aged >70 (63%) and 171 were <70 (65%) (p = 0.93).
High RS was a hallmark of high clinical risk tumors (77% of all high RS cases were also of high clinical risk) and also a predictor of high clinical risk in older patient. However, when analyzing CHT implications, clinical risk did not influence the decision to enroll older patients in CHT regimens and this also remained true for younger patients.
Older patients in our population were treated more often with radical surgeries, including axillary dissection, endocrine monotherapy, and less systemic therapy. They underwent less often reconstructive surgical interventions, and consequently had a lower rate of surgical complications, which is usually associated with reconstruction, vs younger patients. They had more osteo-oncologic treatment, less RT and less CHT in all RS categories. Since more radical initial approaches and fewer systemic therapies were performed in the older cohort, local recurrence was lower when compared to distant failure. Older patients demonstrated a tendency toward higher recurrence rates, which appear to be significant in patients with RS >25. Per our statistical analysis, treatment administration was not determined by age, clinical risk, or tumor biology, although older patients received standardized treatment less often than younger patients. Although the Oncotype DX RS remains the only National Comprehensive Cancer Network (NCCN)-recommended signature test in older patients [8], this segment of population experience a clear gap in accessing standard care for various reasons. In our study, older patients in the intermediate and high RS categories received less often CHT in comparison to younger patients from the same categories (3% and 47% vs. 16% and 84% p = 0.013).
Since 3.1% of older patients were assigned to CHT in the intermediate RS category we believe that physicians considered CHT according to TAILORx subgroup analysis results which showed significant benefit of CHT in the RS category 16–25 [9]. Since the results of the RxPONDER study were published in December 2021 [10] and our study concluded in January 2022, we can safely infer that postmenopausal status was probably not considered when triaging patients for CHT.
Our study showed a lack of convergence between the tumor board decision-making and current RS-based guidelines and pointed toward a more personalized treatment approach, especially in older patients.
However, this may indicate compliance of the treatment decision with the RS guidelines at the time of diagnosis. Since the TAILORx results were first published in July 2018, a subsequent analysis focused on data collected until July 2018 could shed light on the clinical implementation of the guidelines.
Cox regression analysis showed no prognostic value of RS with regards to overall survival (OS). However, statistical analysis could have been affected by the lower number of registered events in our population.
The calculated mortality rate in our older patient population was 5%, which is in line with previously published data. A SEER Database study reported that 4.9%, 21.3%, and 3.7% patients with breast cancer died from breast cancer, non-cancer-related events, and a secondary tumor, respectively. All patients had a probability of death as a result of breast cancer, of 3% and 4.7% at 5 and 8 years respectively, whereas the probability of dying due to other causes accounted for 9.8% and 18.9% [11].
Because of our study limitations, we cannot draw relevant conclusions on any benefit of CHT administration in our cohorts. Importantly, all older patients with high RS were referred to chemoendocrine therapy, showing that physicians still tend to adhere to guidelines.
Conclusions
Oncotype DX RS in older patients might help to tailor individualized therapy. If patients refuse CHT, they can also forego Oncotype DX® testing. Although RS-based guidelines still apply in therapy decision-making for older patients with breast cancer, clinical practice points towards individualized treatment solutions for each patient, in which all clinical and pathological factors are weighted.
CRediT authorship contribution statement
E.D. Chiru: Writing – original draft, Conceptualization, Methodology, Writing – review & editing. C. Grasic Kuhar: Validation, Software, Writing – review & editing. A. Oseledchyk: Writing – review & editing. A. Schötzau: Software, Writing – review & editing. M.J. Gonzalez: Writing – review & editing. C. Kurzeder: Writing – review & editing. M. Vetter: Validation, Data curation, Writing – original draft, Investigation.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Marcus Vetter reports financial support, administrative support, article publishing charges, and travel were provided by ExactScience. Marcus Vetter reports a relationship with ExactScience that includes: consulting or advisory and funding grants.
All the other authors do not have a conflict of interest.
Acknowledgements
Funding sources: This work was supported by the University Hospital Basel, Kantonsspital Baselland, Switzerland, and Exact Sciences Switzerland.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101724.
Appendix. Supplementary materials
References
- 1.Tamirisa N., L. H. S. Y. S. S. S. K. M. G. S. B. G. B. I Association of chemotherapy with survival in elderly patients with multiple comorbidities and estrogen receptor-positive, node-positive breast cancer. JAMA Oncol. 2020;1(6):1548–1554. doi: 10.1001/jamaoncol.2020.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tahir M., R T.S.A. How not to neglect the care of elderly breast cancer patients? Breast. 2011;20(4):293–296. doi: 10.1016/j.Breast.2011.03.003. 2011 AugEpub 2011 May 6. PMID: 21530254. [DOI] [PubMed] [Google Scholar]
- 3.Biganzoli L., B. N. W. H. M. A. C. G. K. I. C. M. C. K. de G. N. T. R. K.-G. B. S.-P.-C. E. P. A. T. J. M. L. B. K. A. M. B. EGC Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG) Lancet Oncol. 2021;22(7):E327–E340. doi: 10.1016/S1470-2045(20)30741-5. 2021 JulEpub 2021 May 14. PMID: 34000244. [DOI] [PubMed] [Google Scholar]
- 4.Kizy S., A A.M., M S., D J.W., J E.H., T T.M., H J.Y.C. 21-gene recurrence score testing in the older population with estrogen receptor-positive breast cancer. J. Geriatr. Oncol. 2018 doi: 10.1016/j.Jgo.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Desai P., Aggarwal A. Breast cancer in women over 65 years- a review of screening and treatment options. Clin. Geriatr. Med. 2021;37(4):611–623. doi: 10.1016/J.CGER.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Bastiaannet E., L G.J., de C A.J.M., et al. Breast cancer in elderly compared to younger patients in the Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res. Treat. 2010;124:801–807. doi: 10.1007/S10549-010-0898-8. (2010) [DOI] [PubMed] [Google Scholar]
- 7.Biskup E., Vetter M., Wedding U. Fighting Diagnostic and therapeutic nihilism in the elderly with cancer. Ann. Palliat. Med. 2020;9(3) doi: 10.21037/apm.2019.08.03. apm.amegroups.com/article/view/28273/html [DOI] [PubMed] [Google Scholar]
- 8.Zhou P., Zhang W.W., Bao Y., Wang J., Lian C.L., He Z.Y., Wu S.G. Chemotherapy and 21-gene recurrence score testing for older breast cancer patients: a competing-risks analysis. Breast. 2020;54:319–327. doi: 10.1016/j.breast.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sparano J.A., C. M. T. G. G. R. S. S. S. S Development and validation of a tool integrating the 21-gene recurrence score and clinical-pathological features to individualize prognosis and prediction of chemotherapy benefit in early breast cancer. J. Clin. Oncol. 2021;39(6):557–564. doi: 10.1200/JCO.20.03007. 2021 Feb 20Epub 2020 Dec 11. PMID: 33306425; PMCID: PMC8078482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalinsky K., Barlow W.E., Gralow J.R., Meric-Bernstam F., Albain K.S., Hayes D.F., Lin N.U., Perez E.A., Goldstein L.J., Chia S.K.L., Dhesy-Thind S., Rastogi P., Alba E., Delaloge S., Martin M., Kelly C.M., Ruiz-Borrego M., Gil-Gil M., Arce-Salinas C.H., Hortobagyi G.N. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. New England J. Med. 2021;385(25):2336. doi: 10.1056/NEJMOA2108873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasif N., Neville M., Gray R., Cronin P., Pockaj B.A. Competing risk of death in elderly patients with newly diagnosed stage i breast cancer. J. Am. Coll. Surg. 2019;229(1):30–36. doi: 10.1016/j.jamcollsurg.2019.03.013. e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.