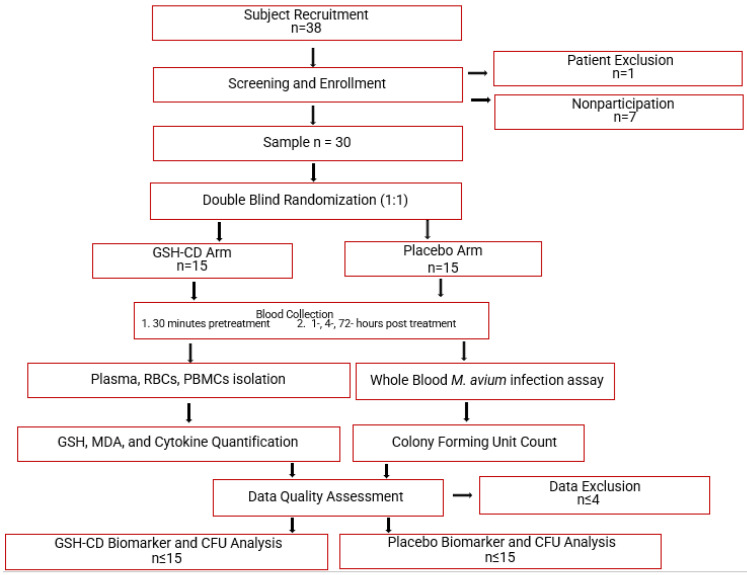
Figure 1.
Topical glutathione cyclodextrin complex clinical trial study design. Subjects (n = 38) were screened for inclusion and exclusion criteria. After screening, 1 participant was excluded due to chronic diabetes and 7 eligible participants did not continue to complete the study before the initial blood draw. Participants (n = 30) were randomly assigned to either the placebo arm (n = 15) or the GSH-CD arm (n = 15) blind to both the participant and investigator. Participants were instructed to apply 4 sprays (0.5 mL) of topical GSH-CD solution (cyclodextrin with 100 mg rGSH) or placebo (cyclodextrin only) on the abdomen twice a day for 3 days. Blood draws were collected before and after topical solution application at various time points. Blood samples were processed to extract plasma, RBCS, and PBMCs to assess for relevant biomarkers. A portion of the blood sample was used to perform whole blood infection assays. Placebo and treatment arms were compared after data quality assessment and data exclusion.