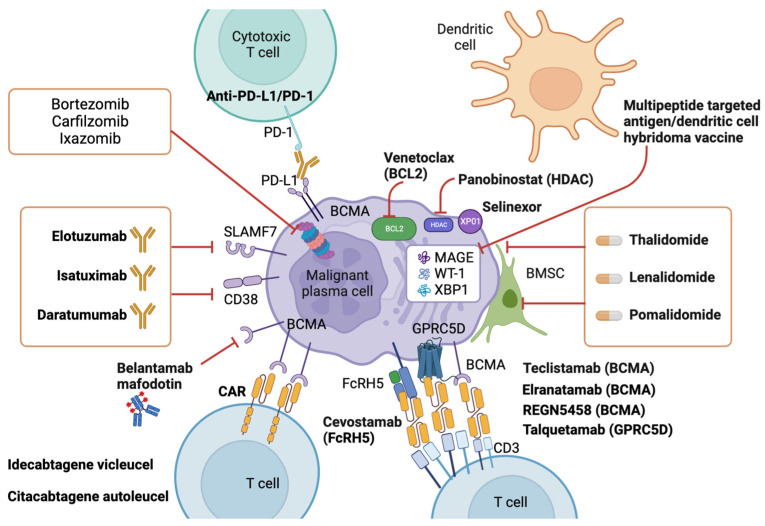
Figure 3.
Modern therapy in multiple myeloma. This figure illustrates the pathomechanisms of modern therapeutic approaches for multiple myeloma (MM), which target key pathways involved in MM pathogenesis and progression. The figure highlights the following therapeutic strategies: (i) proteasome inhibitors: Proteasome inhibitors, such as bortezomib and carfilzomib, inhibit the activity of the proteasome complex, leading to the accumulation of misfolded proteins and induction of apoptosis in MM cells. (ii) Immunomodulatory drugs: Immunomodulatory drugs, such as lenalidomide and pomalidomide, modulate the immune microenvironment in MM by inhibiting the production of pro-inflammatory cytokines, enhancing T cell function, and promoting natural killer cell activity. (iii) Monoclonal antibodies: Monoclonal antibodies, such as daratumumab and elotuzumab, target specific antigens on MM cells, leading to their destruction through antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). (iv) Cell-based therapies: Cell-based therapies, such as chimeric antigen receptor (CAR) T cell therapy, involve the engineering of T cells to express CARs that recognize and kill MM cells (v) Targeted therapies: Targeted therapies, such as inhibitors of the phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway or the B-cell lymphoma-2 (BCL-2), histone deacetylases (HDAC) and Exportin-1 (XPO1) family of proteins, target specific signaling pathways or molecules that are dysregulated in MM cells, leading to their inhibition and apoptosis. MAGE, WT-1, and XBP1 are important targets in myeloma research, offering potential avenues for novel therapies. MAGE (Melanoma-Associated Antigen) and WT-1 (Wilms Tumor 1) are cancer-testis antigens often overexpressed in multiple myeloma, making them attractive targets for immunotherapies like cancer vaccines and adoptive T-cell therapies. Targeting these antigens aims to induce an immune response specifically against myeloma cells, sparing healthy tissues. Additionally, XBP1 (X-Box Binding Protein 1) is a transcription factor critical for plasma cell differentiation and survival. Inhibiting XBP1 holds promise as a therapeutic strategy to disrupt the survival mechanisms of myeloma cells, potentially leading to improved treatment outcomes. Research focusing on these targets shows great potential in advancing precision medicine approaches for multiple myeloma. Finally, novel immunological targeting strategies are represented. Teclistamab: a promising therapy that targets B-cell maturation antigen (BCMA), a cell surface protein highly expressed on multiple myeloma cells. Teclistamab is designed to direct the immune system to attack BCMA-expressing myeloma cells. Elranatamab: an investigational therapy also targeting BCMA, aiming to trigger the immune system to eliminate myeloma cells expressing this antigen. Elranatamab holds potential as a novel treatment for multiple myeloma. REGN5458: another BCMA-targeting therapy that seeks to harness the immune system to target and destroy BCMA-expressing myeloma cells. REGN5458 represents an exciting advancement in the field of multiple myeloma treatment. Talquetamab: an innovative therapy that targets G protein-coupled receptor family C group 5 member D (GPRC5D), a protein found on the surface of myeloma cells. Talquetamab aims to engage the immune system in attacking GPRC5D-expressing myeloma cells. Cevostamab: a potential therapeutic option that targets Fc receptor homolog 5 (FcRH5), a cell surface protein expressed on myeloma cells. Cevostamab aims to induce an immune response against FcRH5-expressing myeloma cells. Idecabtagene vicleucel: an innovative approach using chimeric antigen receptor (CAR) T-cell therapy, specifically Idecabtagene vicleucel, to target and eliminate multiple myeloma cells. This personalized treatment involves modifying patients’ own T-cells to express a CAR that recognizes and attacks myeloma cells. Citacabtagene autoleucel (Cita-cel) is another chimeric antigen receptor (CAR) T-cell therapy used in the treatment of multiple myeloma. It involves engineering a patient’s T-cells to express a CAR that targets BCMA. Understanding the patho-biological mechanism of modern therapeutic approaches for MM is crucial for the development of effective treatment strategies alone and in combination with already approved agents that can improve patient outcomes. Combination therapies that target multiple pathways and mechanisms may offer the best chance for achieving durable responses and long-term disease control in MM [30]. More details are provided in the text. Created by BioRender, publication license n. CW25N085PL.