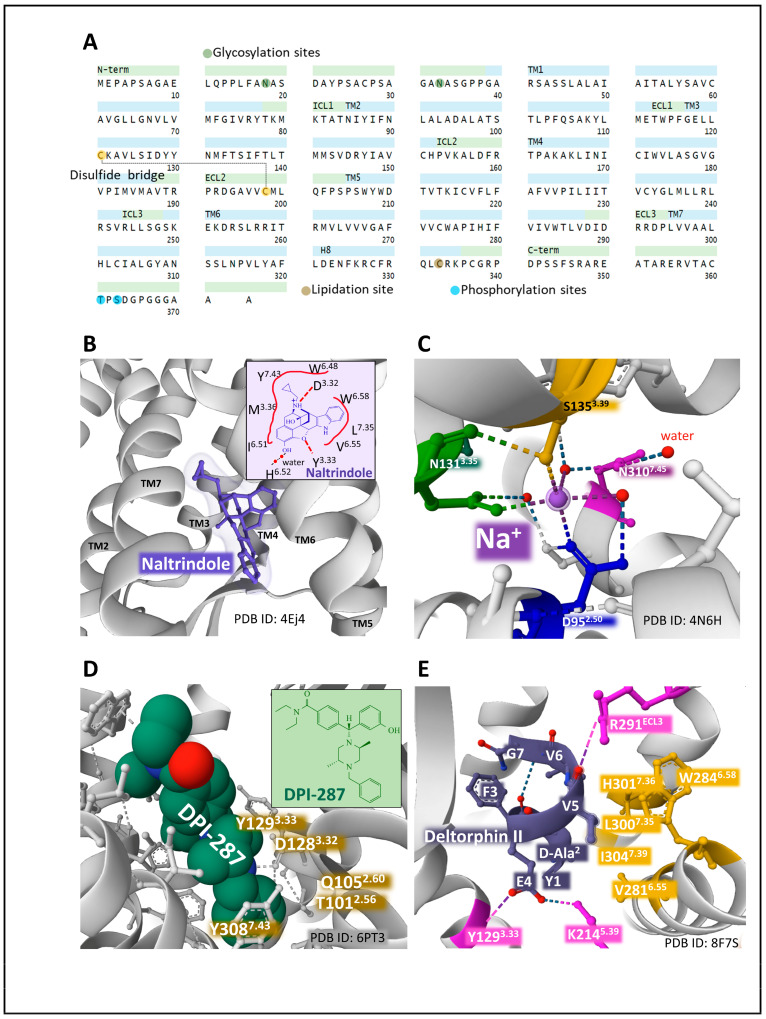
Figure 5.
The primary structure and domains of the DOP receptor with the indication of posttranslational modifications (A) [19,49,50]. The structure of murine DOP bound to the antagonist naltrindole (B) and the 2D structure of naltrindole depicting close interactions with DOP amino acid residues (B, inset). (C) represents the allosteric sodium site of human DOP and the contacts established with residues of the receptor. (D) shows the pocket of human DOP for the agonist DPI-287 and the interactions with DOP residues (inset). E highlights the interaction of peptide agonist deltorphin II with human MOP. The interactions of the amino acid residues of the peptide with the receptors illustrate the orthosteric binding cavity (E). Figures obtained from the Protein Data Bank [51] correspond to PDB ID 4EJ4 [64], 4N6H [65], 6PT3 [66], and 8F7S [67] drawn with the free web-based open-source toolkit Molstar (https://molstar.org/ (accessed on 17 May 2023)) [53]. Two-dimensional structures of naltrindole and DPI-287 were drawn with KingDraw software (version 1.1.0) (https://www.kingdraw.cn/en/ (accessed on 17 May 2023)). Amino acid residues are numbered according to Ballesteros and Weinstein’s nomenclature [57].