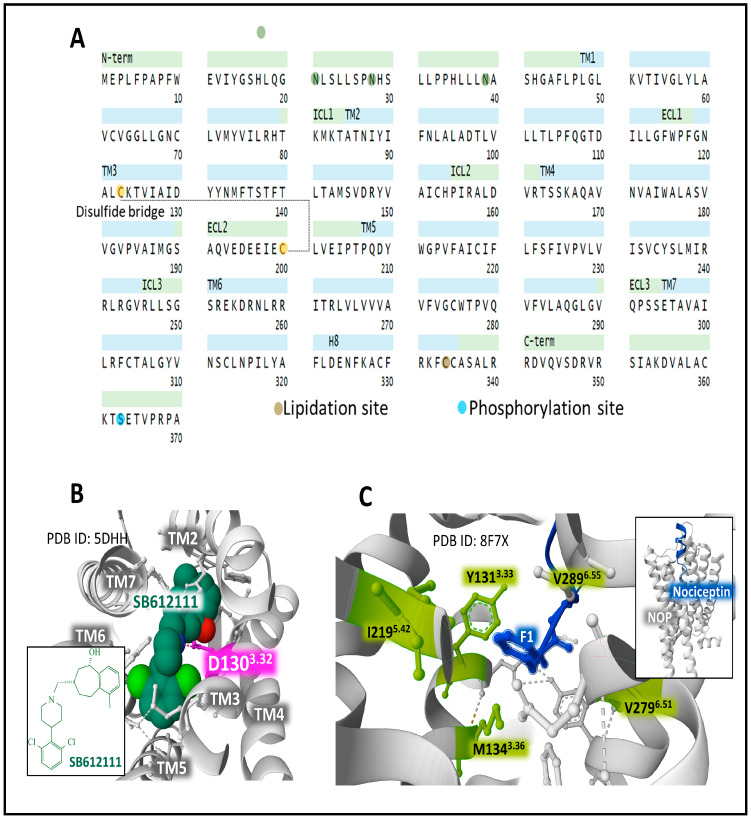
Figure 7.
The primary structure and domains of the NOP receptor with the indication of posttranslational modifications (A) [19,49,50]. The structure of human NOP is bound to the selective antagonist SB612111 ((5S,7S)-7-[[4-(2,6-Dichlorophenyl)piperidin-1-yl]methyl]-1-methyl-6,7,8,9-tetrahydro-5H-benzo [7]annulen-5-ol)) (2D structure in the inset), indicating amino acid contacts in the orthosteric binding pocket (B). The binding nociceptin to NOP (C) reveals the hydrophobic contacts of the ligand with the inferior domain of the binding site delimited by Y1313.33, M1343.36, I2195.42, V2796.51, and V2836.55. The inset figure in C illustrates the position of nociceptin within the orthosteric site of NOP. Figures are from the Protein Data Bank [51] formatting and correspond to PDB ID 5DDH [80] and 8F7X [67], drawn with the free web-based open-source toolkit Molstar (https://molstar.org/ (accessed on 17 May 2023)) [53]. The two-dimensional structure of SB612111 was drawn with KingDraw software (version 1.1.0) (https://www.kingdraw.cn/en/ (accessed on 17 May 2023)). Amino acid residues are numbered according to Ballesteros and Weinstein’s nomenclature [57].