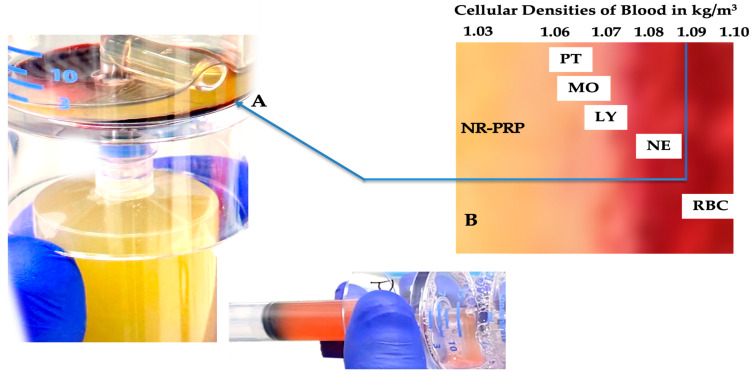
Figure 2.
Whole blood cellular gravitational density separation after a dual-spin procedure. In this picture, a 60 mL PRP device (PurePRP-SP®, EmCyte Corporation, Fort Myers FL, USA, with permission) was used to produce LR-PRP. At A, a thin, gray, multicomponent, buffy-coat layer is visible. Before extraction of this layer, platelets are resuspended in a small volume of plasma and aspirated in a syringe. Resuspending the platelets colors the PRP slightly light amber, indicating a capture of the buffy-coat layer. A magnified image of the buffy-coat layer in B illustrates how blood cells are arranged according to their densities [70]. To realize an LR-PRP specimen, a fraction of RBCs must be part of the bioformulation, as the density of neutrophils overlaps with the density of the RBCs. In this 3.75 mL LR-PRP product, the total available platelets were 10.02 × 109; monocyte and neutrophil concentrations were 7.95 and 9.92 × 103/µL, respectively. The RBC concentration was 2.01 × 106/µL.