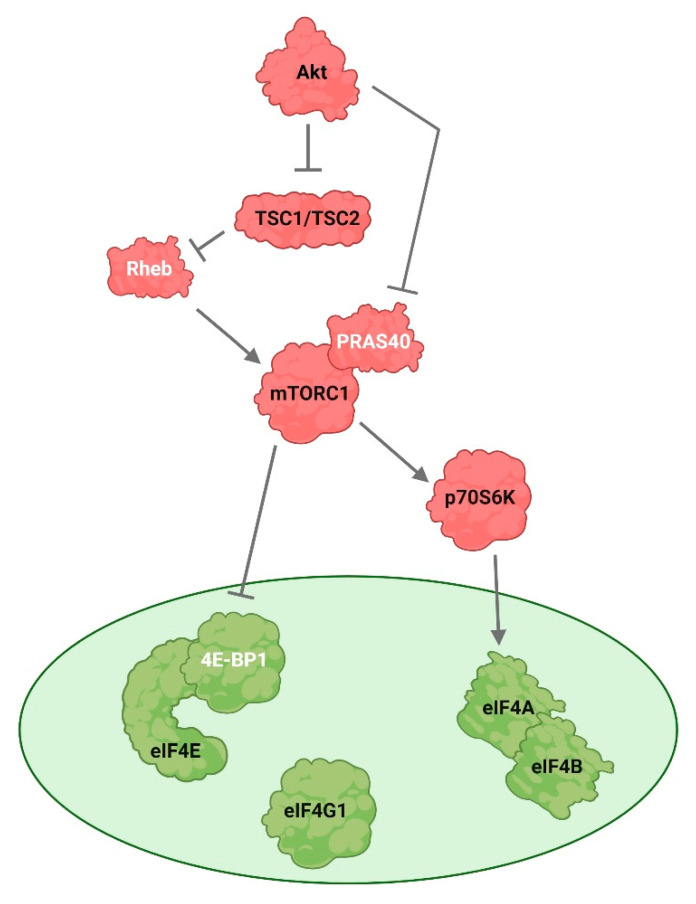
Figure 2.
Akt regulates mRNA translation. Akt directly activates mammalian target of rapamycin complex 1 (mTORC1) by phosphorylating and inactivating proline-rich Akt substrate 40 kDa (PRAS40), a protein that is associated with mTORC1. Further, Akt also activates mTORC1 indirectly by phosphorylating and inactivating the tuberous sclerosis complex 1 (TSC1) and tuberous sclerosis complex 2 (TCS2). TSC1/TSC2 functional complex acts as a key upstream negative regulator of mTORC1 activity. TSC2 has a role as a GTPase-activating protein (GAP) which inactivates an essential mTORC1 activator, the RAS homologue enriched in brain (Rheb). MTORC1 phosphorylates and activates the 70-kDa ribosomal protein S6 kinase (p70S6K), which phosphorylates the eukaryotic translation initiation factor 4B (eIF4B). The eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1), phosphorylated and activated by mTORC1, is released from eIF4E and the assembly of the eIF4F complex is enabled. Factors involved in cap-dependent translation initiation are depicted in green, the upstream factors in red. Stimulatory modification is depicted as an arrow, inhibitory modification as a blunt end line.