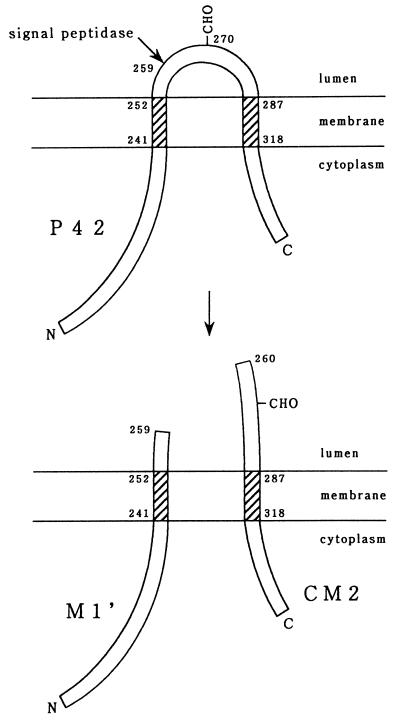
FIG. 5.
Proposed model for the biosynthesis of CM2. The 374-amino-acid protein (P42) has two hydrophobic domains capable of interacting with a lipid bilayer (shaded) and a N-glycosylation site (CHO). This protein is cleaved by signal peptidase after Ala residue 259 to produce the M1′ and CM2 proteins composed of the N-terminal 259 amino acids and C-terminal 115 amino acids, respectively.