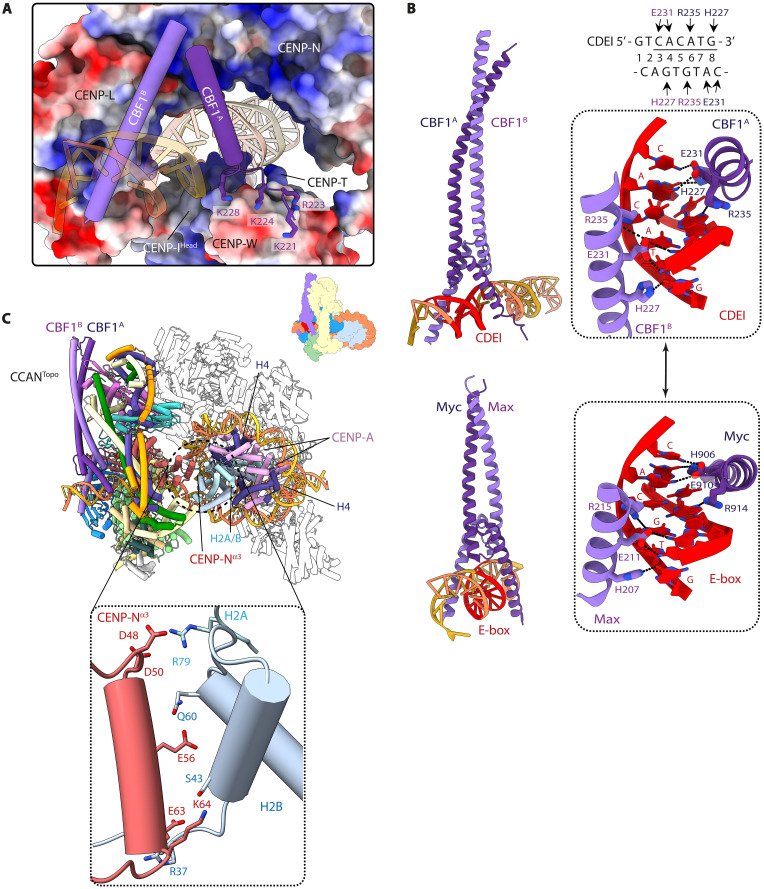
Fig. 3. CBF1 extends the basic DNA binding CENP-LN channel through interactions with CDEI.
(A) The DNA binding tunnel of CCAN has a marked electropositive potential and is extended by the basic α helices of CBF1. An acidic patch on CENP-TW binds basic residues of the CBF1A helix, thereby unfolding the N-terminal half of the helix. (B) The sequence-specific contacts of CBF1 with CAC(A/G)TG of CDEI (top) are nearly identical to how the Myc-Max transcription factor interacts with its cognate E-box CACGTC motif (bottom). (C) Details of how the CENP-N α3 helix interacts with basic residues of histones H2A-H2B that are exposed because of unwrapping of the CENP-ANuc DNA gyre. Hence, 33 bp of C0N3-CENP-ANuc is unwrapped at its 5′ end.