Abstract
Toll-like receptor (TLR) agonists improve vaccine immunogenicity and efficacy, but they are currently unlicensed as adjuvants in influenza vaccines. This study aimed to investigate whether a combination of monophosphoryl lipid A (MPL, a TLR4 agonist) and polyriboinosinic polyribocytidylic acid (poly I:C, a TLR3 agonist) can enhance the protective efficacy of an inactivated A/Puerto Rico/8/1934 (A/PR8) H1N1 influenza vaccine against homologous influenza infection and minimize illness outcomes. Results showed that combination MPL and poly I:C adjuvanted influenza vaccination increased the production of antigen-specific antibodies, decreased the levels of cytokines and cellular infiltrates at the infection sites, and induced significant memory T and B cell responses in mice. The results of this study suggest that the combination of MPL and poly I:C can be developed into a possible adjuvant for enhancing the efficacy of influenza vaccines.
Subject terms: Adaptive immunity, Infectious diseases, Vaccines
Introduction
Influenza infections are responsible for an average of 250,000–500,000 respiratory deaths annually, predominantly caused by the A(H1N1) 2009 pandemic (pdm09) subtype (in people < 65 years) and A(H3N2) subtype viruses (in people ≥ 65 years)1. Vaccination is the most effective strategy for preventing influenza virus infections. Widely used seasonal influenza vaccines are inactivated whole-virus, split-virus, and protein subunit vaccines, but the efficacy of influenza vaccines is suboptimum2–4. Moreover, these vaccines are ineffective in conferring cross-protection against antigenically different influenza viruses; thus, annual influenza vaccination is required, leading to an economic burden on the healthcare system and society4,5.
Innate immune responses to pathogens are commonly induced by the recognition of specific patterns of pathogens via toll-like receptors (TLRs), which are expressed on most innate immune cells. Interactions between TLRs and their ligands activate downstream factors of TLR-dependent signaling pathways, such as nuclear factor-kappa B, activator protein-1, and interferon (IFN) regulatory factor-3/7. The release of pro-inflammatory cytokines and infiltration of innate immune cells into the infection site further initiate antigen-specific adaptive immunity6. Therefore, TLR agonists are promising candidates as vaccine adjuvants to improve vaccine efficacy and enhance mucosal and humoral immunity.
Monophosphoryl lipid A (MPL) is a TLR4 agonist that stimulates immune responses via myeloid differentiation factor 88 (MyD88) and Toll-IL-1R domain-containing adaptor-inducing IFN-β factor (TRIF)-dependent pathways; at high doses (10–50 μg), MPL is used as an adjuvant for respiratory syncytial virus vaccines7. Synthetic double-stranded RNA polyriboinosinic polyribocytidylic acid (poly I:C) is a TLR3 agonist that induces the TRIF-dependent signaling pathway, leading to the release of pro-inflammatory cytokines and local immune responses. Poly I:C-adjuvanted influenza vaccines elicit a high anti-hemagglutinin (HA) response in nasal wash and enhance the production of IgG isotype antibodies, resulting in increased protection against influenza viral infections8. In previous studies, both MPL and poly I:C have been used with a relatively high dose; 10 μg to 50 μg of MPL and 50 μg of Poly I:C for mice, and up to 1 mg per each adjuvant for Rhesus Macaques immunization9–12. A combination adjuvant composed of low doses of MPL (1 μg) and poly I:C (10 μg) positively promotes innate and specific adaptive immune responses to ovalbumin13,14. In this study, we aimed to investigate whether the combination of MPL and poly I:C enhances the protective efficacy of an inactivated A/Puerto Rico/8/1934 (A/PR8) H1N1 influenza vaccine in comparison with nonadjuvanted or individual adjuvanted vaccines after homologous viral challenge and elicit heterosubtypic antibody production against the A/Hong Kong/1/68 (A/HK) H3N2 virus.
Results
MPL + poly I:C-adjuvanted iPR8 vaccine enhances antigen-specific antibody production
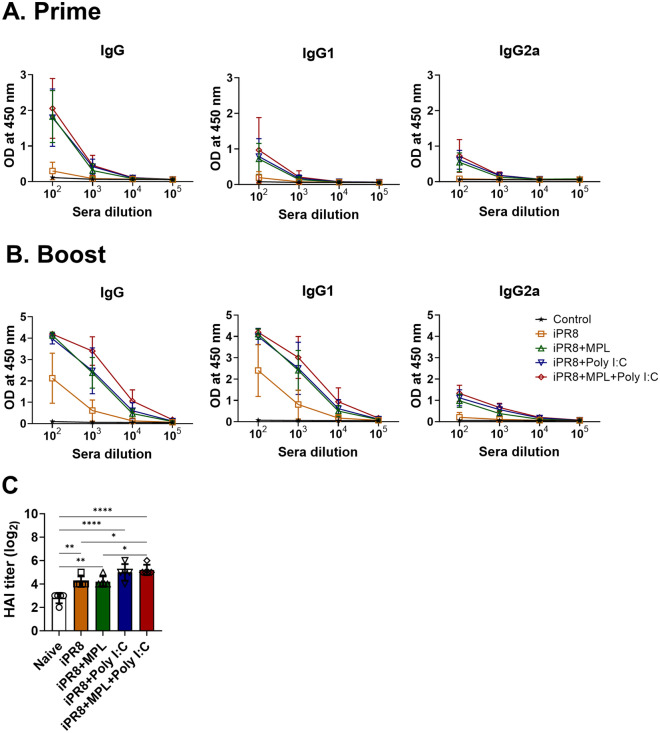
To determine the effects of the adjuvanted influenza vaccines, we intramuscularly injected BALB/c mice (n = 10/group) with an inactivated A/PR8 (H1N1) vaccine (iPR8, 1 μg) alone or in combination with an adjuvant (either MPL 1 μg, poly I:C 10 μg, or MPL 1 μg + poly I:C 10 μg) two times with a 3 weeks interval. Sera were collected at 2 weeks after each immunization, and A/PR8-specific isotype antibodies were measured using ELISA. The A/PR8-specific HAI titers at 2 weeks post boost immunization were determined by performing an HAI assay. Compared with the nonadjuvanted vaccines, the adjuvanted iPR8 vaccines induced higher levels of virus-specific total IgG, IgG1, and IgG2a isotype antibodies after the prime immunization (Fig. 1A). The levels of serum anti-A/PR8 antibodies were significantly increased by boost dose of iPR8 with MPL + poly I:C compared with iPR8 with MPL or poly I:C (Fig. 1B). iPR8 alone vaccination elicited the production of Th2-induced IgG1 isotype antibodies but not Th1-induced IgG2a isotype antibodies after boost immunization. The mice in the MPL- or poly I:C-adjuvanted vaccine group presented a significant increase in the levels of antigen-specific antibodies compared to the unadjuvanted vaccine group. In addition, the HAI titers in immune sera were higher in the mice from the poly I:C- or MPL + poly I:C-adjuvanted group than in the mice from the MPL-adjuvanted or nonadjuvanted group (Fig. 1C).
Figure 1.
Antibody production after inactivated A/PR8 vaccine with or without adjuvants. BALB/c mice (n = 10) were intramuscularly immunized two times with a 3 weeks interval, with inactivated A/PR8 (1 μg) with or without adjuvants (1 μg of MPL, 10 μg of poly I:C or MPL 1 μg + poly I:C 10 μg). (A and B) A/PR8 (H1N1)-specific antibody levels in sera collected at 2 weeks after prime and boost immunizations, respectively. (C) Hemagglutination inhibition (HAI) titers of boost sera against homologous A/PR8 (H1N1). All results are presented as mean ± standard deviation (SD) with individual dots. Statistical analysis was performed using two-way ANOVA and Tukey’s post-multiple comparison tests. *P < 0.0332, **P < 0.0021, ****P < 0.0001.
MPL + poly I:C-adjuvanted iPR8 vaccine enhances protection against the lethal homologous influenza infection
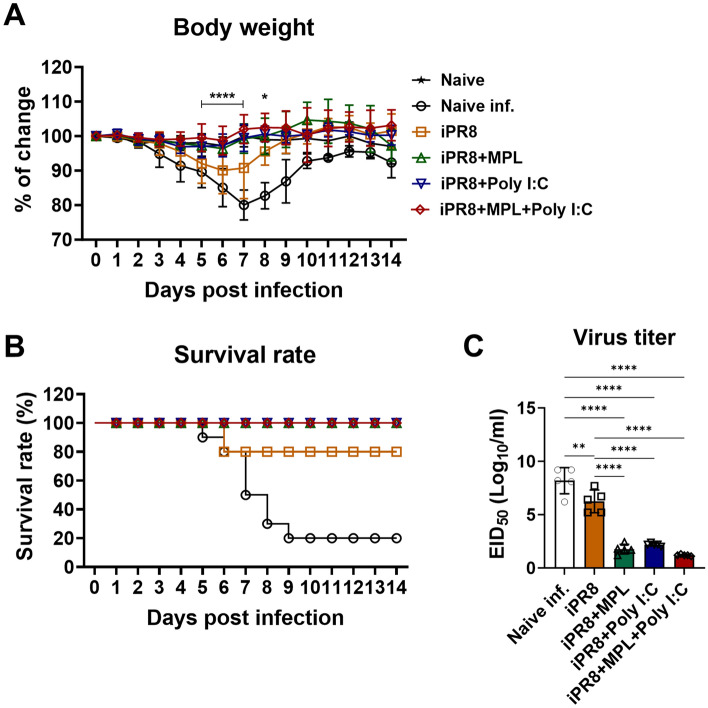
At 3 weeks after boost vaccination, the naïve and vaccinated mice were challenged with a lethal dose of A/PR8 (1.5 × LD50) to investigate the protective efficacy of the vaccine with the adjuvants. To rule out the non-specific protective effects of adjuvant itself, mice were immunized with adjuvants only (MPL, Poly I:C, or MPL + Poly I:C without iPR8) and evaluated antibody production and protection against the virus challenge. The adjuvant only immunization could not provide anti-viral responses as well as any antigen-specific antibody production (Supplementary Fig. 1). The adjuvanted vaccines showed effective protection against a lethal dose of A/PR8 infection, as evidenced by the no loss in body weight (BW) and 100% survival rate of the challenged mice, which is similar to those of the uninfected naïve mice. However, the naïve mice displayed severe weight loss (19%–25%) at 7 days post challenge (dpi) and 20% survival rate (Fig. 2A,B). Even survived mice exhibited severe illness such as near 20% weight loss, hunched posture, and scruffy hair coat during the infection. The iPR8-vaccinated mice displayed a slight decrease in BW (10–20%) and 80% survival rate at 6 days post challenge. After 9 days of challenge, the weights of the iPR8-vaccinated mice recovered to normal, but those of the naïve infected mice did not (Fig. 2A,B).
Figure 2.
Protective efficacy of the adjuvanted vaccines in the immunized mice after the lethal influenza virus challenge. Immunized BALB/c mice (n = 10) were challenged with a lethal dose of homologous A/PR8 (1.5 × LD50) after 3 weeks of boost immunization. Body weight (BW) (A) and survival rates (B) of the challenged mice (n = 5) were measured for 14 days after viral infection, and percentages of changes were calculated based on BW at day 0. For statistical analysis, two-way ANOVA and Tukey’s post-multiple comparison tests were performed. *p < 0.0332, and ****p < 0.0001 between iPR8 and iPR8 + MPL + Poly I:C groups. (C) Lung homogenates (n = 5) were harvested at day 7 post infection, and lung viral titers were measured. Statistical analysis was performed using one-way ANOVA and Tukey’s post-multiple comparison tests. **P < 0.0021, ***P < 0.0002, ****P < 0.0001. All results were shown in mean ± standard deviation (SD) with individual dots.
To investigate whether immunization with inactivated influenza vaccine together with adjuvants could prevent viral replication, we collected lung samples of vaccinated mice at 7 days after homologous challenge to measure viral titers (Fig. 2C). The nonvaccinated and iPR8-only-vaccinated mice showed significantly high levels of lung viral titers at 7 dpi, whereas the mice in the single-adjuvanted and MPL + poly I:C-adjuvanted groups exhibited significantly lower titers, suggesting better viral control in the lungs. In particular, the mice in the MPL + poly I:C-adjuvanted group showed the detection limit of viral titers. These data suggest that the MPL- and poly I:C-adjuvanted iPR8 vaccines provided complete protection against homologous infection.
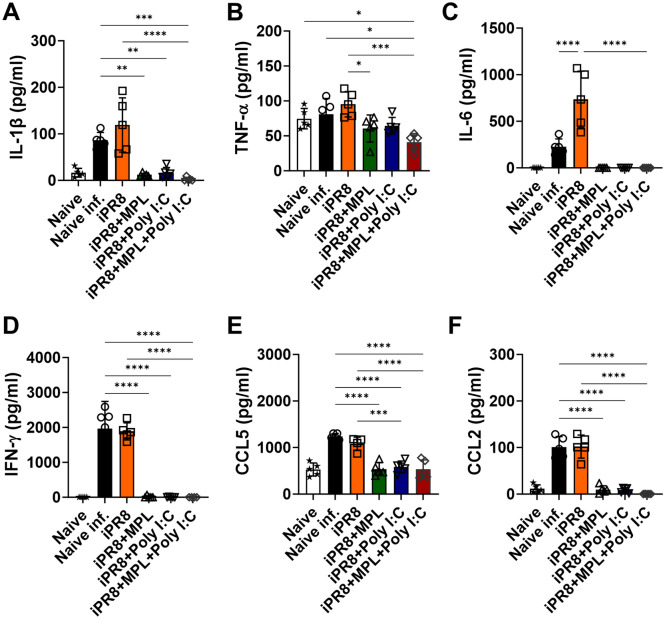
Cytokine storms with high levels of pro-inflammatory cytokines elicit inflammatory cell recruitment to the infection site; however, the cytokine levels in the lungs after infection are inversely correlated with viral loads in the lungs and positively correlated with disease severity during influenza infection15,16. We measured the levels of cytokines and chemokines in lung extracts harvested from the mice in the vaccinated groups at 7 dpi (Fig. 3). Among the mice in this study, the naïve infected and iPR8-only-vaccinated mice showed the highest production of cytokines (IL-1β, TNF-α, IL-6, and IFN-γ) and chemokines (CCL2 and CCL5) after lethal viral challenge. The adjuvanted immunizations, however, exhibited low or no cytokine/chemokine production, indicating that the adjuvants increased the protective efficacy of the vaccine.
Figure 3.
Cytokines and chemokines released into lung extracts in BALB/c mice after homologous A/PR8 challenge. Lung samples of the vaccinated BALB/c mice (n = 5) were harvested at 7 days post challenge. Cytokine and chemokine levels were measured using ELISA. All results are presented as mean ± standard deviation (SD) with individual dots. Statistical analysis was performed using one-way ANOVA and Tukey’s post-multiple comparison tests. *P < 0.0332, **P < 0.0021, ***P < 0.0002, ****P < 0.0001.
To further confirm the protective efficacy of the adjuvant combination against the influenza virus infection, lung samples were harvested, processed, and stained with hematoxylin and eosin. Severe lung inflammation was observed in naïve and iPR8 immunized groups 7 days post virus challenge. Meanwhile MPL- and poly I:C-adjuanted groups exhibited moderate levels of lung inflammation, MPL + poly I:C-adjuvanted group did not display lung histopathology (Supplementary Fig. 2).
MPL + poly I:C-adjuvanted iPR8 vaccine prevents inflammatory cell infiltrates after A/PR8 viral infection
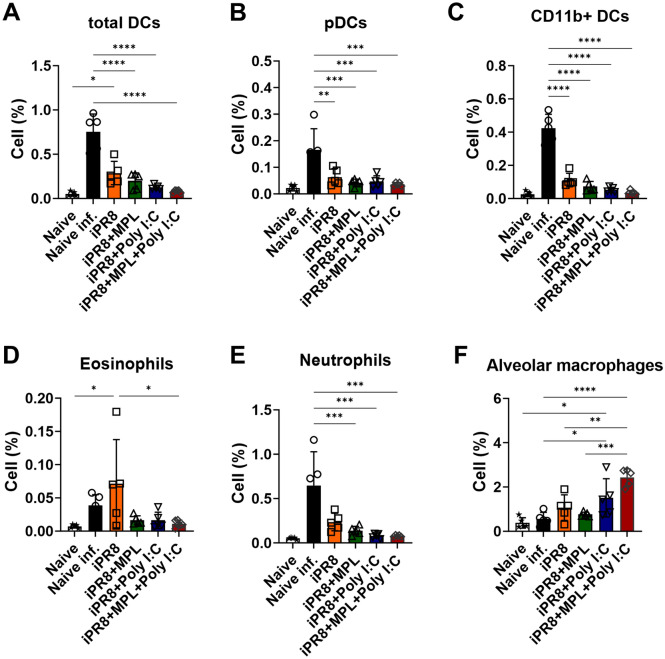
Cellular infiltrates in the lungs of immunized BALB/c mice were observed 7 days after homologous viral challenge to assess a possible correlation with the protective efficacy of the vaccines (Fig. 4). Inflammatory cells play a role in protection against invading pathogens, but they also contribute to lung inflammation, leading to severe illness outcomes upon viral infection17,18. Consistent with the high production of pro-inflammatory cytokines (Fig. 3), inflammatory cells, such as eosinophils, neutrophils, and DCs were recruited to the lungs of the naïve mice after viral infection. More inflammatory cells were observed in the lungs of the mice from the single-adjuvanted groups than in those of the mice from the MPL + poly I:C-adjuvanted group, but the difference was not significant (Fig. 4A–E). Alveolar macrophages (AMs) are the first line of defense against pathogens invading the airway. The percentages of AMs were maintained in the mice from the iPR8-only, poly I:C-adjuvanted, and MPL + poly I:C-adjuvanted groups, whereas the mice from the naïve infected and MPL-adjuvanted groups showed decreased AM populations in the lungs (Fig. 4F). These data suggest that the MPL + poly I:C adjuvant in the inactivated vaccine effectively prevented the infiltration of inflammatory cells into the lungs upon influenza infection.
Figure 4.
Inflammatory cell infiltration into the lungs after homologous viral infection. Immunized BALB/c mice (n = 5) were infected with A/PR8 (1.5 × LD50) after 3 weeks of boost immunization. Lung samples were harvested at day 7 post infection and then cell phenotypes were investigated by flow cytometry. All results were shown in mean ± standard deviation (SD) with individual dots. Statistical analysis was performed using one-way ANOVA and Tukey’s post-multiple comparison tests. **P < 0.0021, ***P < 0.0002, ****P < 0.0001.
Induction of memory T cell populations and antigen-specific T-cell responses as well as memory B cell responses by the intramuscular administration of vaccine adjuvanted with MPL + poly I:C
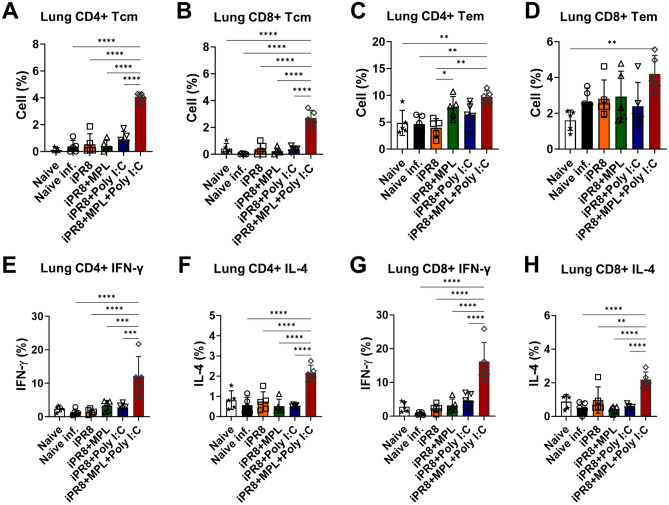
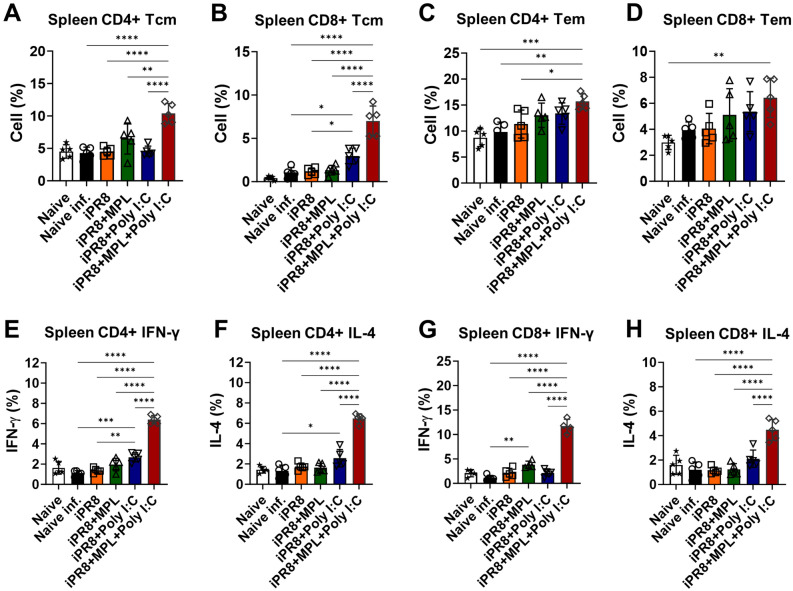
Memory T cells provide protective functions against the invading influenza virus, and high frequencies of antigen-specific memory T cells contribute to the less severe lung diseases19,20. Furthermore, after resolving viral invasion, the majority of effector T cells die, leaving a small proportion of long-lived memory T cells contributing to the prevention of reinfection21. To test the hypothesis that adjuvants can influence the populations of memory T cells, we examined memory T cells and antigen-specific T cell responses at 7 dpi. The adjuvanted vaccine groups after virus exposure increased the populations of effector memory CD4/CD8 T cells (TEM) in the lungs and spleens (Figs. 5C,D and 6C,D), but MPL + poly I:C-adjuvanted vaccination strongly increased the populations of central memory CD4/CD8 T cells (TCM) (Figs. 5A,B and 6A,B). Subsequently, we examined the levels of IFN-γ/IL-4-producing T cell populations in the lungs and spleens of the immunized or naïve mice at 7 dpi after homologous A/PR8 antigen stimulation in vitro. Similarly, the cytokine production of T cells was higher in the mice from the MPL + poly I:C-adjuvanted group after antigen stimulation than in the mice from the other groups (Figs. 5E–H and 6E–H).
Figure 5.
Memory T cell populations and iPR8-specific IFN-γ and IL-4-producing T cells in the lungs of immunized mice. Vaccinated mice were infected with a lethal dose of A/PR8 (1.5 × LD50) after 3 weeks of vaccination. Lung samples were harvested 7 days after virus challenge and then stimulated with inactivated PR8 antigen for 5 h. Cell phenotypes were evaluated using flow cytometry. All results are presented as mean ± standard deviation (SD) with individual dots. Statistical analysis was performed using one-way ANOVA and Tukey’s post-multiple comparison tests. **P < 0.0021, ***P < 0.0002, ****P < 0.0001.
Figure 6.
Splenic memory T cell populations and iPR8-specific IFN-γ and IL-4-producing T cells in the immunized mice. Vaccinated mice were infected with a lethal dose of A/PR8 (1.5 × LD50) after 3 weeks of vaccination. Splenocytes were harvested 7 days after viral challenge and then stimulated with inactivated PR8 antigen for 5 h. All results are expressed as mean ± standard deviation (SD) with individual dots. Statistical analysis was performed using one-way ANOVA and Tukey’s post-multiple comparison tests. *P < 0.0332, **P < 0.0021, ***P < 0.0002, ****P < 0.0001.
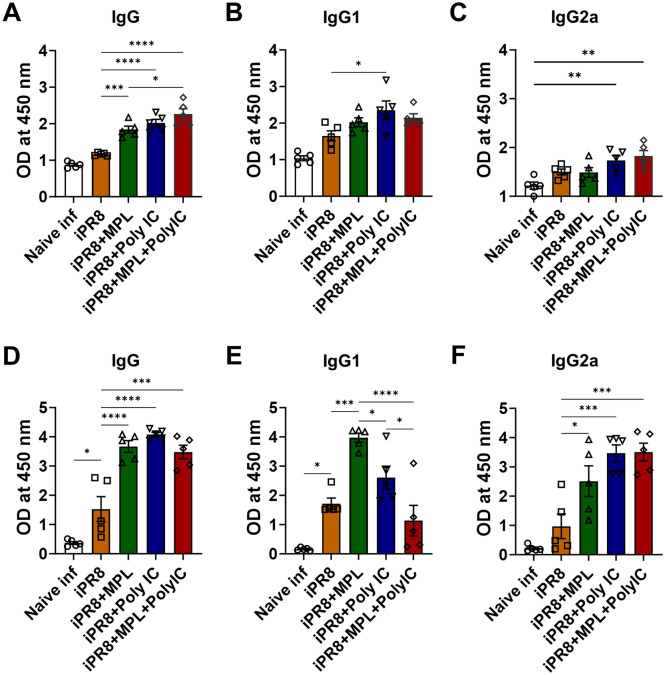
To investigate antibody-producing plasma and memory B cell responses induced by the adjuvanted vaccination, bone marrow and spleen cells were harvested and cultured in antigen-coated plates. MPL + poly I:C-adjuvanted group exhibited significantly enhanced IgG production with higher IgG2a/IgG1 ratio (Fig. 7).
Figure 7.
Antigen-specific antibody production of bone marrow and spleen cells from the immunized mice. Bone marrow (A–C) and spleen cells (D–F) were harvested at day 7 post virus infection and cultured in iPR8-coated plates for 1 day or 7 days, respectively. IgG, IgG1, and IgG2a ELISA was performed to measure the antigen-specific antibody production. All results are expressed as mean ± standard deviation (SD) with individual dots. Statistical analysis was performed using one-way ANOVA and Tukey’s post-multiple comparison tests. *P < 0.0332, **P < 0.0021, ***P < 0.0002, ****P < 0.0001.
Collectively, these results suggest that the MPL + poly I:C-adjuvanted vaccine was effective in eliciting memory T-cell differentiation and inducing antigen-specific functional T cell populations in addition to memory B cell responses, which provided an efficient protective response against viral invasion.
Discussion
Apart from COVID-19, influenza infections are among the most urgent infectious diseases to date and responsible for thousands of deaths annually worldwide. Vaccination is a common strategy for preventing the influenza pandemic. Inactivated pathogen vaccines are safer than live attenuated vaccines. Nevertheless, inactivated whole-virus vaccines sometimes lose immunogenicity and do not efficiently induce cellular adaptive immunity22. Adjuvants have been introduced to effectively stimulate vaccine antigen-specific immune responses via different pathogen-associated molecular pattern signaling pathways. TLR agonists are widely used as adjuvants that stimulate immune responses by regulating TRIF or MyD88-dependent signals23–25. MPL is an attenuated type of lipopolysaccharide that stimulates immune responses by upregulating TRIF/MyD88-dependent signaling pathways, which are licensed for hepatitis B virus vaccine (Fendrix) and human papillomavirus vaccine (Cervarix)7,26. Poly I:C is a synthetic double-stranded RNA that effectively triggers immune responses to the influenza virus by promoting the TRIF-dependent signaling pathway, which has been tested in humans8,26. The combination of MPL and poly I:C can induce strong innate immune responses and further stimulate antigen-specific antibody production and antigen-specific T cell responses to ovalbumin at high levels13,14. In the present study, the inactivated influenza virus (inactivated A/PR8 (H1N1)) vaccine combined with either MPL, poly I:C, or MPL + poly I:C at low doses exerted additive effects on enhancing virus-specific IgG, IgG1, and IgG2a isotype antibody production; preventing weight loss disease, pro-inflammatory cytokine release and inflammatory immune cell infiltration into the infection site upon viral infection. Additionally, the MPL + poly I:C-adjuvanted iPR8 vaccine elicited robust memory immunity, as demonstrated by the increased levels of antigen-specific antibody production by plasma and memory B cells, frequencies of central/effector memory T cells and antigen-specific cytokine producing memory T cell responses in the secondary lymphoid organs. These results suggest that the inactivated vaccine adjuvanted with the combination of TLR3 and 4 agonists (MPL and poly I:C, respectively) showed effective protection against viral infection with better antigen-specific memory responses.
In the present study, the adjuvanted groups showed strong humoral immune responses with high levels of virus-specific antibodies (Fig. 1A,B). This result might be related to the pre-existence of memory CD4/CD8 T cell populations in the lungs and spleens upon viral infection21,27. In addition to serum antibody responses, neutralizing antibody (NAb) binding to HA plays a vital role in preventing the interaction between virus sialic acid receptors and the host cell27–31. The MPL + poly I:C- or individual poly I:C-adjuvanted iPR8 was effective in inducing high levels of HAI titers against A/PR8 (H1N1) (Fig. 1C), which contributed to viral clearance upon infection, as demonstrated by the very low viral titers (Fig. 2C). Moreover, NAbs not only function against the same strain of influenza but also accelerate the clearance of different serotypes of influenza32. Thus, we determined the A/HK (H3N2)-specific IgG and HAI titers in the sera and IgG production by plasma cells derived from bone marrow cells after immunization and homologous virus challenge (Supplementary Fig. 3). The MPL + poly I:C-adjuvanted vaccine significantly enhanced heterosubtypic A/HK (H3N2)-specific immune responses such as binding IgG, HAI activities, and antibody-secreting plasma cells, suggesting that the combination adjuvant may elicit cross-protection against different influenza strains.
The inflammatory cytokines and chemokines released into infection sites play vital roles in the recruitment of immune cells against invading pathogens33. However, excessive recruitment of cell infiltrates and high levels of pro-inflammatory cytokines contribute to immune-mediated damage, which exacerbates illness outcomes due to influenza infection34,35. AMs provide a crucial defense against pathogens during infection; lung-resident AMs contribute to the protection of alveoli from inflammation following viral infection36. In the present study, high levels of lung-resident AMs and moderately low frequencies of inflammatory infiltrates into the lungs were observed in the mice from the MPL + poly I:C-adjuvanted group after influenza infection (Fig. 4), and these mice had no BW losses and 100% survival rates (Fig. 2A,B).
An efficient response of the Th1 and Th2 subsets contributes to the control of viral infections. However, high levels of IL-4, correlated with the induction of the Th2 response, may delay viral clearance37. Eosinophils, which are recruited in response to IL-4 and Th2 expression, have been associated with local allergic responses that can damage lung histopathology21,37. Though virus infection usually induces Th1 immune responses due to their intracellular activity, inactivated influenza virus vaccine was previously reported to induce Th2-biased immune responses38,39. As a result, immunization of whole-virus inactivated vaccine may present serious adverse events and lower vaccine efficacy. In the present study, the inactivated vaccine adjuvanted with the combination of MPL and poly I:C provided enhanced protection and reduced inflammation at the infection site (Fig. 2), but it also possibly increased Th1-polarized immune responses, as evidenced by the increased levels of antigen-specific IFN-γ and IL-4 (Fig. 5E–H), which provided sufficient protection against illness severity due to viral infection40.
Lung-resident memory CD8 T cells are primarily localized in the epithelial layers and can mediate rapid responses to invading pathogens. Additionally, lung-resident memory T cells mainly comprise tissue-resident memory T cells (TRM cells), which play a predominant role in protective immunity41. Among functional CD8 T cell subsets, effector CD8 T cells protect against influenza by blocking viral replication. High levels of CD8+IFN-γ+ were reported to be correlated with an elimination of symptoms, reduction of illness severity, and clearance of virus in infected patients19,42–44. Aside from CD8 T cells, memory CD4 T cells contribute directly to viral clearance by interacting with antigen-presenting cells, helping virus-specific antibody-producing B cells, and improving CD8 T cell functionality21. CD4 T cell-derived IFN-γ directly downregulates T-bet expression on CD8 T cells, leading to TRM formation during influenza infection45. Moreover, IL-4-producing T cells induce the differentiation of CD44highCD62Lhigh central memory cell-like CD8 T cells after viral infection, which is a robust effector cytokine that controls chronic viral infection; therefore, virus-specific IL-4-induced innate CD8 T cells are potentially used in T-cell therapy46. In the present study, the MPL + poly I:C-adjuvanted iPR8 vaccine induced higher levels of CD8/CD4 TCM and TEM residing in the lungs compared with the individual adjuvanted and nonadjuvanted vaccines (Fig. 5), which conferred complete protection from severe disease outcomes upon viral infection. Furthermore, the vaccine with the combination adjuvant elicited more functional T-cell immunity, such as antigen-specific IFN-γ and IL-4 production, than the other vaccines. In addition to the homologous protection, local CD4 and CD8 T cells confer cross-protection against different serotypes of influenza viruses47, supporting the cross-protective efficacy of the MPL + poly I:C-adjuvanted vaccine.
T cells residing in secondary lymphoid tissues are important for providing long-lasting antiviral responses. Most effector CD4/CD8 T cells die after viral infection, but only a small number of memory T cells persist in the long term at the infection site48–50. Therefore, the memory CD4/CD8 T cells located in secondary lymphoid organs appear to play a critical role in the early clearance of the virus upon reinfection. In addition, central memory T cells have a unique functional attribute of migrating to the infection sites following differentiation into effector T cells producing antiviral cytokines, resulting in rapid and effective antiviral responses due to virus reinfection51. Our data revealed that the MPL + poly I:C-adjuvanted vaccine was not only effective in enhancing splenic TCM and TEM cell populations but also in efficiently inducing T cells to release significantly high levels of antigen-specific effector cytokines (IL-4 and IFN-γ).
In summary, the results of this study showed that the iPR8 vaccine adjuvanted with the combination of MPL and poly I:C enhanced antigen-specific antibody production, memory T cell responses, and protection against homologous influenza infection. In addition, the MPL + poly I:C-adjuvanted vaccine showed potential cross-reactive IgG and HAI activities. Thus, this study suggests the combination of MPL and poly I:C as an adjuvant for influenza vaccines. However, further studies are warranted to elucidate the mechanisms by which the MPL + poly I:C adjuvant induces cross-protection.
Methods
Mice and reagents
Female BALB/c mice (n = 5 each group, OrientBio Co., Gyeonggi, Korea) were used in this study. The mice were 8–10 weeks old at the time of priming immunization. The mice were fed standard chow diet, provided with water ad libitum, and maintained under a 12 h light/dark cycle at 22 ± 2 °C. The mice were anesthetized with isoflurane and O2 gas mixture for the experimental procedures. All animal experiments confirmed to the International Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of Jeju National University, South Korea (protocol number: 2021–0051). All animal experiments were conducted in accordance with relevant regulations and guidelines, and reported in accordance with ARRIVE guidelines.
MPL and poly I:C were purchased from InvivoGen (San Diego, CA). All reagents were prepared in accordance with the manufacturer’s instructions. A/PR8 and A/HK grown in the allantoic cavity of embryonated chicken eggs were used to prepare an inactivated virus vaccine with 1% neutral buffered formalin overnight at 4 °C and then concentrated by ultracentrifugation (30,000 rpm, 1 h). The inactivated virus pellets were resuspended in phosphate-based saline (PBS) and then stored at − 80 °C until use. Live A/PR8 was used as the homologous challenge.
Immunization and viral infection
BALB/c mice (n = 10/group) were immunized intramuscularly with inactivated PR8 (iPR8) vaccine (1 μg) alone or in combination with MPL (1 μg), poly I:C (10 μg), or MPL + poly I:C (1 μg + 10 μg, respectively). Vaccinations were administered two times (prime and boost) with a 3 weeks interval, and sera were collected 2 weeks after each immunization. At 6 weeks after the last immunization, the naïve and vaccinated mice were challenged intranasally with a lethal dose (1.5 × LD50) of A/PR8. Changes in body weight (BW) and survival rates were monitored for 14 days after the challenge. In accordance with IACUC guidelines, mice displaying more than 20% BW loss were considered to have reached the endpoint and humanely euthanized to minimize pain.
Enzyme-linked immunosorbent assay for antibody and cytokine productions
Immune sera were collected 2 weeks after each vaccination, and antigen-specific antibody levels were determined using enzyme-linked immunosorbent assay (ELISA). ELISA plates were coated with either inactivated A/PR8 (600 ng/well) or A/HK (H3N2) overnight at 4 °C and blocked with PBS containing 3% bovine serum albumin and 0.05% tween20. The plates were added with serially diluted immune sera, incubated, and then washed. Horseradish peroxidase-labeled secondary antibodies were used to detect antigen-specific IgG, IgG1, and IgG2a antibodies. Tetramethylbenzidine was used as a substrate, and absorbance was obtained at an optical density of (OD) 450 nm by using an ELISA reader.
The levels of cytokines and chemokines in lung extracts obtained from immunized mice were measured using interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α kits from Invitrogen (Waltham, MA, USA) and interferon IFN-γ, monocyte chemoattractant protein-1 (CCL2/MCP-1), and C–C motif chemokine ligand 5 (CCL5) kits from R&D Systems (Minneapolis, MN, USA) in accordance with the manufacturers’ instructions.
Lung viral titration
Lungs of the A/PR8-infected BALB/c mice were harvested at 7 days postinfection (dpi) and minced mechanically with 2 mL of Roswell Park Memorial Institute (RPMI) 1640 medium (Fisher Scientific, Corning, NY, USA) per lung. Lung extracts were collected by centrifugation, and 200 μl of extracts was saved for measurement of lung viral titers. Embryonated chicken eggs were prepared for 9–10 days to be inoculated with diluted lung extracts with PBS, and viral titers were determined by HA assay of the allantoic fluids collected after 3 days of incubation. Viral titers at 50% egg infection dose (EID50)/ml were evaluated using the Reed and Muench method52.
HA inhibition (HAI) assay
The ability of immune sera to inhibit HA was estimated by performing an HAI assay after boost and challenge infection. Immune sera collected from immunized BALB/c mice were inactivated by incubation at 56 °C for 30 min and then treated with the receptor-destroying enzyme (RDE, Sigma-Aldrich) for 18 h at 37 °C. Subsequently, sera were serially diluted in PBS and incubated with 4 HA units of A/PR8 (H1N1) or 4 HA units of A/HK (H3N2) for 30 min at room temperature (RT), and then 0.5% chicken red blood cells were added to determine HAI titers.
Memory B cell response
The A/PR8 or A/HK (H3N2)-coated plates were blocked with 10% fetal bovine serum containing RPMI 1640 medium for 1 h at RT to measure the memory B cell response to influenza virus. Bone marrow and spleen cells derived from the infected mice at 7 dpi were seeded at a density of 2 × 106 cells/ml onto the plates and then incubated at 37 °C for 1 day or 5 days, respectively. Anti-mouse IgG, IgG1, IgG2a antibodies were used to detect influenza-specific antibodies secreted by antibody producing B cells.
Flow cytometry
The inflammatory cell infiltration in the respiratory tract was investigated by collecting lung samples at 7 dpi and staining single cells with antibodies specific for CD45 (clone 30-F11), CD3 (clone 17A2), CD11b (clone M1/70), CD11c (clone N418), F4/80 (clone BM8), Ly6c (clone AL-21), major histocompatibility complex (MHC) class II (clone I-A/I-E), SiglecF (clone E50-2440), B220 (RA3-6B2), and live/dead-amcyan (LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit) after blocking the Fc receptor.
Lungs and spleens were harvested and stained with antibodies specific for CD45, CD3, CD4 (clone RM4.5), CD8a (clone 53-6.7), CD44 (clone IM7), CD62L (clone MEL-14), and live/dead cells to explore the memory T cells in lung and spleen cells.
Intracellular cytokines (IL-4 [clone 11B11] and IFN-γ [clone XMG1.2]) of CD4 and CD8 T cells residing in the lung and spleen cells were stained after 5 h stimulation with inactivated A/PR8 to estimate the A/PR8 specific-IL-4/IFN-γ production by CD4 and CD8 T cells. Golgi stop (monensin, a protein transport inhibitor) was added to each sample during the stimulation. Intracellular cytokine staining was performed using the fixation/permeabilization solution kit (BD Biosciences) in accordance with the manufacturer’s instructions.
The phenotypes of the acquired cells were analyzed and gated by marker expressions on cells: alveolar macrophages (AMs): CD45+CD11b−CD11c+F4/80+; neutrophils: CD45+CD11b+Ly6clowF4/80−; eosinophils: CD45+CD11b+SiglecF+; total dendritic cells (DCs): CD45+F4/80−CD11c+MHCIIhigh; plasmacytoid DCs (pDCs): CD45+F4/80−CD11c+MHCIIhighB220+; CD11b+DCs: CD45+F4/80−CD11c+MHCIIhighCD11b+; CD4 naïve T cell: CD45+CD3+CD4+CD44−CD62L+; CD8 naïve T cell: CD45+CD3+CD8+CD44−CD62L+; CD4 central memory T cell (TCM): CD45+CD3+CD4+CD44+CD62L+; CD8 TCM: CD45+CD3+CD8+CD44+CD62L+; CD4 effector memory T cell (TEM): CD45+CD3+CD4+CD44+CD62Lˉ; CD8 TEM: CD45+CD3+CD8+CD44+CD62Lˉ. The gating strategies of inflammatory cells and T cells are shown in Supplementary Figs. 4 and 5.
Statistical analysis
All results are presented as the mean ± standard error of the mean (SEM). Statistical significance was determined using 1-way ANOVA or 2-way ANOVA and considered at P < 0.05. All data were analyzed using GraphPad Prism statistical software Inc. (San Diego, CA, USA).
Supplementary Information
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2020R1F1A1073040, E.J.K.), Basic Science Research Program to Research Institute for Basic Sciences (RIBS) of Jeju National University through the NRF funded by the Ministry of Education (2019R1A6A1A10072987, E.J.K.) and NIH/NIAID grant (AI154656, S.M.K.).
Author contributions
Conceptualization and design: C.T.T.L., E.J.K.; Data generation and collection: C.T.T.L., S.Y.A., T.L.H., J.L., D.H.L.; Data Analysis: C.T.T.L., S.Y.A., T.L.H., E.J.K.; Data interpretation: C.T.T.L., S.Y.A., T.L.H., J.L., D.H.L., H.S.H., S.M.K., E.J.K.; Writing: C.T.T.L, E.J.K.; Review and editing: C.T.T.L, H.S.H., S.M.K., E.J.K.; Supervision: H.S.H., S.M.K., E.J.K.; All authors contributed significantly to the work, reviewed and finalized the manuscript.
Data availability
All relevant data generated during this study are available upon reasonable request made to correspondence authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hye Suk Hwang, Email: hshwnag33@scnu.ac.kr.
Sang-Moo Kang, Email: skang24@gsu.edu.
Eun-Ju Ko, Email: eunju@jejunu.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-39210-6.
References
- 1.Paget J, et al. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health. 2019;9:020421–020421. doi: 10.7189/jogh.09.020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollard AJ, Bijker EMJNRI. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J. Clin. Microbiol. 1986;24:157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domínguez A, Godoy P, Torner N. The effectiveness of influenza vaccination in different groups. Expert Rev. Vaccines. 2016;15:751–764. doi: 10.1586/14760584.2016.1142878. [DOI] [PubMed] [Google Scholar]
- 5.Putri WCWS, Muscatello DJ, Stockwell MS, Newall AT. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36:3960–3966. doi: 10.1016/j.vaccine.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 7.Blanco JCG, Boukhvalova MS, Pletneva LM, Shirey KA, Vogel SN. A recombinant anchorless respiratory syncytial virus (RSV) fusion (F) protein/monophosphoryl lipid A (MPL) vaccine protects against RSV-induced replication and lung pathology. Vaccine. 2014;32:1495–1500. doi: 10.1016/j.vaccine.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichinohe T, et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 2005;79:2910–2919. doi: 10.1128/jvi.79.5.2910-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apostolico JS, et al. Poly(I:C) potentiates T cell immunity to a dendritic cell targeted HIV-multiepitope vaccine. Front. Immunol. 2019;10:843. doi: 10.3389/fimmu.2019.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park H, et al. Polyinosinic-polycytidylic acid is the most effective TLR adjuvant for SIV Gag protein-induced T cell responses in nonhuman primates. J. Immunol. 2013;190:4103–4115. doi: 10.4049/jimmunol.1202958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLeod MK, et al. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7914–7919. doi: 10.1073/pnas.1104588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravindran R, Bhowmick S, Das A, Ali N. Comparison of BCG, MPL and cationic liposome adjuvant systems in leishmanial antigen vaccine formulations against murine visceral leishmaniasis. BMC Microbiol. 2010;10:181. doi: 10.1186/1471-2180-10-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le CTT, Ahn SY, Kang S-M, Ko E-J. Functional NK cell activation by ovalbumin immunization with a monophosphoryl lipid A and poly I: C combination adjuvant promoted dendritic cell maturation. Vaccines. 2021;9:1061. doi: 10.3390/vaccines9101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn SY, Le CTT, Ko E-J. Monophosphoryl lipid A and poly I: C combination adjuvant promoted ovalbumin-specific cell mediated immunity in mice model. Biology. 2021;10:908. doi: 10.3390/biology10090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beigel J, et al. Writing committee of the world health organization (WHO) consultation on human influenza A/H5. Avian Influ. A (H5N1) Infect. Hum. 2005;4:1374–1385. [Google Scholar]
- 16.Ichiyama T, et al. Cerebrospinal fluid and serum levels of cytokines and soluble tumor necrosis factor receptor in influenza virus-associated encephalopathy. Scand. J. Infect. Dis. 2003;35:59–61. doi: 10.1080/0036554021000026986. [DOI] [PubMed] [Google Scholar]
- 17.Yokota S, et al. Hypothetical pathophysiology of acute encephalopathy and encephalitis related to influenza virus infection and hypothermia therapy. Pediatr. Int. 2000;42:197–203. doi: 10.1046/j.1442-200x.2000.01204.x. [DOI] [PubMed] [Google Scholar]
- 18.Hussell T, Goulding J. Structured regulation of inflammation during respiratory viral infection. Lancet Infect. Dis. 2010;10:360–366. doi: 10.1016/s1473-3099(10)70067-0. [DOI] [PubMed] [Google Scholar]
- 19.Sridhar S, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013;19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 20.Moyron-Quiroz JE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. npj Vaccines. 2021;6:28. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 24.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed SS, Plotkin SA, Black S, Coffman RL. Assessing the safety of adjuvanted vaccines. Sci. Transl. Med. 2011;3:9392. doi: 10.1126/scitranslmed.3002302. [DOI] [PubMed] [Google Scholar]
- 26.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radbruch A, et al. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 28.Eisen HN, Siskind GW. Variations in affinities of antibodies during the immune response*. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 29.Smith KGC, Light A, Nossal GJV, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knossow M, et al. Mechanism of neutralization of influenza virus infectivity by antibodies. Virology. 2002;302:294–298. doi: 10.1006/viro.2002.1625. [DOI] [PubMed] [Google Scholar]
- 31.Lee C-C, et al. An effective neutralizing antibody against influenza virus H1N1 from human B cells. Sci. Rep. 2019;9:4546. doi: 10.1038/s41598-019-40937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallewaard NL, et al. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell. 2016;166:596–608. doi: 10.1016/j.cell.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang JM, An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q, Zhou Y-H, Yang Z-Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol. Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokota S, et al. Hypothetical pathophysiology of acute encephalopathy and encephalitis related to influenza virus infection and hypothermia therapy. Pediatr. Int. Off. J. Jpn Pediatr. Soc. 2000;42:197–203. doi: 10.1046/j.1442-200x.2000.01204.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith AM, Smith AP. A critical, nonlinear threshold dictates bacterial invasion and initial kinetics during influenza. Sci. Rep. 2016;6:38703. doi: 10.1038/srep38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran TM, Isobe H, Fernandez-Sesma A, Schulman JL. Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J. Virol. 1996;70:5230–5235. doi: 10.1128/jvi.70.8.5230-5235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleebrahim-Dehkordi E, et al. T helper type (Th1/Th2) responses to SARS-CoV-2 and influenza A (H1N1) virus: From cytokines produced to immune responses. Transpl. Immunol. 2022;70:101495. doi: 10.1016/j.trim.2021.101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J. Infect. Dis. 1999;180:579–585. doi: 10.1086/314952%JTheJournalofInfectiousDiseases. [DOI] [PubMed] [Google Scholar]
- 40.Rubinstein MP, Cole DJJTII. CD4+ T cells and IL-4-mediated support of Th1 immune responses. Trends Immunol. 2002;23:280. doi: 10.1016/S1471-4906(02)02220-2. [DOI] [Google Scholar]
- 41.Takamura S, Kohlmeier JE. Establishment and maintenance of conventional and circulation-driven lung-resident memory CD8+ T cells following respiratory virus infections. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalvani A, et al. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859%JJournalofExperimentalMedicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruder D, Srikiatkhachorn A, Enelow RI. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006;19:147–155. doi: 10.1089/vim.2006.19.147. [DOI] [PubMed] [Google Scholar]
- 44.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. Production of interferon-γ by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am. J. Pathol. 2001;158:119–130. doi: 10.1016/S0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laidlaw BJ, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41:633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee A, et al. IL-4 induced innate CD8+ T cells control persistent viral infection. PLoS Pathog. 2015;11:e1005193–e1005193. doi: 10.1371/journal.ppat.1005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee Y-T, et al. Intranasal vaccination with M2e5x virus-like particles induces humoral and cellular immune responses conferring cross-protection against heterosubtypic influenza viruses. PLoS ONE. 2018;13:e0190868. doi: 10.1371/journal.pone.0190868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis M, Tarlton JF, Cose SJI. Memory versus naive T-cell migration. Immunol. Cell Biol. 2008;86:226–231. doi: 10.1038/sj.icb.7100132. [DOI] [PubMed] [Google Scholar]
- 49.Masopust D, Schenkel JMJNRI. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 50.Von Andrian UH, Mackay CRJNEJOM. T-cell function and migration—two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 51.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 52.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938;27:493–497. doi: 10.1093/oxfordjournals.aje.a118408%JAmericanJournalofEpidemiology. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data generated during this study are available upon reasonable request made to correspondence authors.