Abstract
Since their discovery nearly five decades ago, molecular scaffolds belonging to the 14-3-3 protein family have been recognized as pleiotropic regulators of diverse cellular and physiological functions. With their ability to bind to proteins harboring specific serine and threonine phosphorylation motifs, 14-3-3 proteins can interact with and influence the function of docking proteins, enzymes, transcription factors, and transporters that have essential roles in metabolism and glucose homeostasis. Here, we will discuss the regulatory functions of 14-3-3 proteins that will be of great interest to the fields of metabolism, pancreatic β-cell biology, and diabetes. We first describe how 14-3-3 proteins play a central role in glucose and lipid homeostasis by modulating key pathways of glucose uptake, glycolysis, oxidative phosphorylation, and adipogenesis. This is followed by a discussion of the contributions of 14-3-3 proteins to calcium-dependent exocytosis and how this relates to insulin secretion from β-cells. As 14-3-3 proteins are major modulators of apoptosis and cell cycle progression, we will explore if 14-3-3 proteins represent a viable target for promoting β-cell regeneration and discuss the feasibility of targeting 14-3-3 proteins to treat metabolic diseases such as diabetes.
Article Highlights
14-3-3 proteins are ubiquitously expressed scaffolds with multiple roles in glucose homeostasis and metabolism.
14-3-3ζ regulates adipogenesis via distinct mechanisms and is required for postnatal adiposity and adipocyte function.
14-3-3ζ controls glucose-stimulated insulin secretion from pancreatic β-cells by regulating mitochondrial function and ATP synthesis as well as facilitating cross talk between β-cells and α-cells.
Introduction
Molecular scaffold proteins belonging to the 14-3-3 protein family are widely conserved among eukaryotes (1–3), and in mammals, they consist of seven ubiquitously expressed isoforms, namely, β, γ, ζ, η, θ, σ, and ε (4). 14-3-3 proteins are 30-kDa acidic proteins that exist as homodimers or heterodimers, and they interact with client proteins through the recognition of specific binding motifs created by phosphorylated serine (Ser) or threonine (Thr) residues. These binding motifs are generally defined by RSXpS/TXP and RXXXpS/TXP, where pS/T represents a phosphorylated serine or threonine residue (5). However, they can also interact with nonphosphorylated proteins (6) or those with other posttranslational modifications, such as O-GlcNAcylation (7). Additionally, 14-3-3 proteins are also regulated by phosphorylation, which promotes their dissociation from client proteins (8,9), the destabilization of 14-3-3 protein homo- or heterodimers (10), or ubiquitination and subsequent degradation (11).
14-3-3 proteins bind to a diverse array of client proteins (enzymes, transcription factors, transporters, etc.) to regulate their compartmentalization or activity, and this results in 14-3-3 proteins being able to influence a broad variety of cellular pathways. This perspective will focus on pathways related to metabolism and glucose homeostasis, calcium-dependent exocytosis, cell cycle regulation and proliferation, and apoptosis and cell survival (Fig. 1). Emphasis will be placed on the relevance of 14-3-3 protein–dependent actions on lipid and glucose metabolism as well as for pancreatic β-cell function and survival. Lastly, the potential or likelihood of 14-3-3ζ to serve as a molecular target for the treatment of metabolic disorders, such as obesity and diabetes, will be discussed.
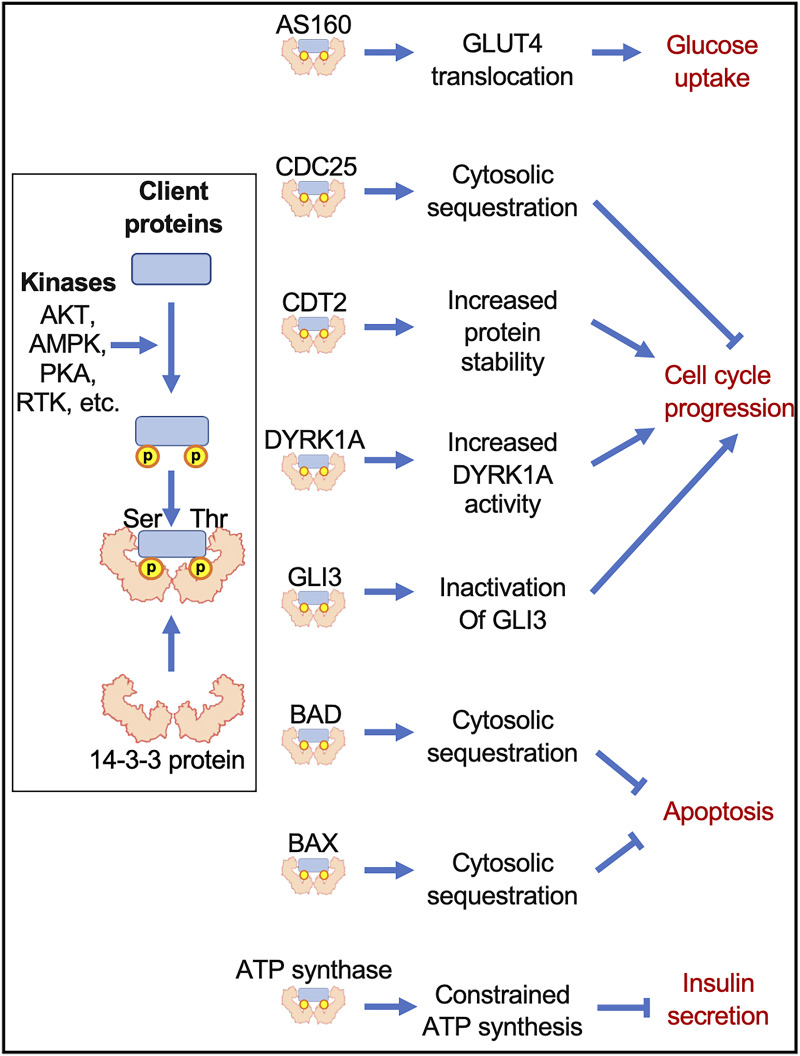
Figure 1.
Pathways regulated by 14-3-3 proteins that influence cell survival and development. 14-3-3 proteins primarily interact with client proteins through the recognition of high-affinity, specific binding motifs created by phosphorylated serine (Ser) or threonine (Thr) residues. This permits 14-3-3 proteins to directly modulate the activity, subcellular localization, and/or stability of a wide range of enzymes, transcription factors, and molecular scaffolds. The latter are key effectors of essential cellular events such as glucose metabolism, cell cycle, apoptosis, or insulin secretion.
Fundamental Roles of 14-3-3 Proteins in the Regulation of Glucose Homeostasis
While generally underappreciated in the context of metabolic regulation, the involvement of 14-3-3 proteins in the control of glucose homeostasis has been documented, as they participate in signal transduction pathways that facilitate glucose uptake. For example, insulin-stimulated phosphorylation of Thr649 on the Rab GTPase-activating protein (RabGAP), AKT substrate of 160 kDa (AS160/TBC1D4), promotes its binding to 14-3-3 proteins, which inhibits the RabGAP activity of AS160 and permits GLUT4 translocation to the plasma membrane (Fig. 1) (12). The importance of this interaction was shown in mice overexpressing an AS160 variant with a Thr649Ala mutation, as impaired glucose uptake, glucose clearance, and insulin sensitivity were observed (12). A similar mechanism in skeletal muscle in response to insulin or contraction has been observed whereby binding of 14-3-3 proteins to GARNL1/RalGAPα1 controls Ral-A–mediated GLUT4 translocation to the plasma membrane (13). Another RabGAP, TBC1D1, governs GLUT4 translocation once phosphorylated by AMP-activated protein kinase (AMPK) on Ser231, and as with Thr649 on AS160, this serine residue is a critical binding site for 14-3-3 proteins (14). The importance of Ser231 in AMPK-mediated glucose uptake was seen in animals expressing a Ser231Ala TBC1D1 mutant, which led to impaired muscle- and whole-body glucose clearance in response to AICAR, a potent activator of AMPK, and loss of GLUT4 translocation in isolated skeletal muscle (15).
Another potential mechanism by which 14-3-3 proteins may affect glucose homeostasis is through their binding to Insulin Receptor Substrate 1 and 2 (IRS1 and IRS2) (16). 14-3-3 proteins bind to IRS1 within its phosphotyrosine binding domain, which attenuates its activation by the insulin receptor (16). 14-3-3 proteins also control the stability of IRS2 through Ser1137- and Ser1138-dependent binding, as observed in HEK293 cells and primary murine hepatocytes (17). In vivo evidence of a role of 14-3-3 proteins in glucose homeostasis was seen through our use of systemic 14-3-3ζ knockout mice, which displayed decreased insulin sensitivity in response to an insulin bolus (18). In a similar vein, decreased 14-3-3ζ abundance in human skeletal muscle biopsies is associated with decreased insulin sensitivity (19). Altogether, these observations demonstrate contributions of 14-3-3 proteins to insulin signaling and glucose homeostasis.
14-3-3ζ Is Crucial for Adipocyte Fate
To date, we have explored the critical roles of 14-3-3ζ in the regulation of whole-body adiposity, adipogenesis, and adipocyte function. Using in vitro and in vivo models, we identified 14-3-3ζ as an essential regulator of adipogenesis (18). Systemic deletion of 14-3-3ζ in mice significantly reduced visceral white adiposity, and glucose intolerance and insulin resistance were observed (18). In contrast, transgenic overexpression of 14-3-3ζ enhanced age- and high-fat-diet–induced weight gain and fat accumulation (18). Mechanistically, 14-3-3ζ was required for mitotic clonal expansion of murine preadipocytes by controlling the abundance of the cyclin-dependent kinase inhibitor CDKN1B/p27Kip1 in addition to stabilizing the abundance of the early adipogenic transcription factor C/EBP-δ during the initial stages of adipogenesis (18).
Fully differentiated white adipocytes also require 14-3-3ζ for their optimal function. Indeed, we discovered that depletion of 14-3-3ζ in mature adipocytes impaired β-adrenergic receptor-mediated lipolysis, one of the principal metabolic functions of adipose tissue (20). This is consistent with previous findings reporting cAMP-dependent protein kinase (PKA)–dependent phosphorylation of Ser660 and Ser406 of hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL), respectively, both of which represent 14-3-3 binding sites (21,22). Phosphorylated HSL and ATGL, together with monoacylglycerol lipase (MGL), catalyze the sequential hydrolysis of triglycerides stored within lipid droplets into diglycerides and free fatty acids and glycerol during lipolysis (23). Depletion of 14-3-3ζ also decreased the expression of peroxisome proliferator–activated receptor γ2 (PPARγ2), which is often considered the master regulator of adipogenesis (20). We also discovered that 14-3-3ζ overexpression enhanced cold-induced beiging of inguinal white adipose tissue, as seen by increased expression of the thermogenic protein Uncoupling Protein 1 (UCP1) (24).
Recently, we identified an alternative mechanism of 14-3-3ζ–dependent adipogenesis (25). In mouse embryonic fibroblasts undergoing adipogenesis, the interactome of a tandem affinity purification (TAP) epitope–tagged 14-3-3ζ was identified by mass spectrometry. Several RNA-splicing factors, such as HNRPF, DDX6, and SFPQ, were enriched in the TAP–14-3-3ζ interactome during adipogenesis, and their silencing via siRNA impeded adipogenesis by preventing the generation of pro-adipogenic mRNA splice variants (25).
Taken together, these findings demonstrate important physiological roles of 14-3-3ζ in adipocyte development and function. In-depth studies aimed at defining the adipocyte-specific contributions of 14-3-3ζ to age- and high-fat-diet–associated weight gain and obesity are still required, and these studies would aid in fully understanding the importance of 14-3-3ζ to adipocyte biology.
The Importance of 14-3-3 Proteins for β-Cell Function, Survival, and Proliferation
The importance of the pancreatic β-cell in the regulation of glucose homeostasis is undeniable, as it is the predominant cell type that produces insulin (Fig. 2A). Impaired insulin secretion and β-cell apoptosis are key determinants in the development of diabetes, which further underscores the importance of the pancreatic β-cell (26). While 14-3-3 proteins are important for regulating metabolic signaling pathways that underpin glucose or lipid homeostasis in various cell types, an important question is whether 14-3-3ζ and its related isoforms similarly influence important aspects of β-cell function, namely, insulin secretion and proliferation.
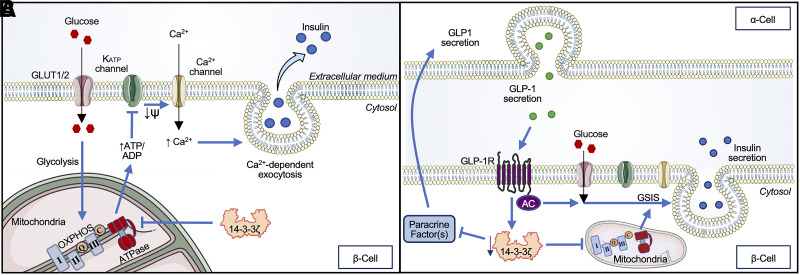
Figure 2.
Role of 14-3-3ζ proteins in exocytosis and insulin secretion. A: The localization of 14-3-3ζ in mitochondria may directly affect ATP synthesis, as evidenced by the ability of 14-3-3ζ to restrict mitochondrial OXPHOS. This ultimately constrains ATP-dependent insulin secretion. B: In parallel, β-cell 14-3-3ζ may regulate cross talk between β-cells and α-cells. Secretion of the insulinotropic peptide GLP-1 by α-cells leads to activation of the GLP-1R in β-cells, leading to reductions in 14-3-3ζ expression and the liberation of β-cell–derived paracrine factors that enhance β-cell function.
14-3-3 Proteins and Cell Cycle Progression Control
In multiple cell types, 14-3-3 proteins are known to regulate the eukaryotic cell cycle (Fig. 1), consisting of growth or Gap1 (G1), DNA synthesis (S), G2, and mitosis (M) phases (27). The transition from each phase is strictly controlled by cyclins (Cyc) and serine/threonine cyclin-dependent kinases (CDKs). Mitogenic stimuli trigger the expression of CycD during G1, which binds to CDKs like CDK4 and CDK6. These CycD-CDK complexes alleviate the inhibition of E2F transcription factors by pocket proteins, thereby promoting the expression of genes involved in S phase. The transitions from S to G2 phases, and then from G2 to M, are driven by CycA-CDK2 and CycB-CDK1 complexes, respectively (27). At key transitions of the cell cycle, Cyc-CDK complexes can be inhibited by CDK inhibitors such as CDKN1B/p27Kip1. Tumor suppressors, such as p53, DNA damage, starvation, or differentiation can induce the expression of various CDK inhibitors, for instance, CDKN1B/p27Kip1, to limit cell cycle progression (18,27).
One of the first pieces of evidence of a regulatory role of 14-3-3 proteins on the cell cycle was reported in colorectal cancer cells and yeast (28). 14-3-3σ, the expression of which is induced by p53, was shown to bind to and sequester the phosphatase Cdc25C in the cytosol, thereby preventing the dephosphorylation of CycB-CDK1 complexes and permitting the transition from G2 to M phase (Fig. 1) (28). The importance of 14-3-3 proteins in cell cycle progression was further described by pan-inhibition or isoform-specific overexpression, which caused aberrant cell cycle progression or blocked proliferation, respectively (29,30). Some 14-3-3 protein isoforms, such as 14-3-3σ, bind to CDK2, CDK4, and various cyclins to inhibit their function and impair cell cycle entry and proliferation (30). Deletion of 14-3-3ε downregulates CycE1 expression, which promotes CDKN1B/p27Kip1 accumulation and prevents progression from G0/G1 to G2/M (31). We have similarly discovered that 14-3-3ζ is essential to the cell cycle during adipogenesis in vitro, as 14-3-3ζ depletion activated a novel GLI3–CDKN1B/p27Kip1 axis to block cell cycle progression in 3T3-L1 preadipocytes (Fig. 1) (18). Alternatively, 14-3-3ε and 14-3-3γ isoforms interact with chromatin licensing and DNA replication factor-2 (CDT2) in a Thr464 phosphorylation–dependent manner to protect CDT2 against E3 ubiquitin ligase–triggered proteasomal degradation (32). This conserves the ability of CDT2 to promote G2/M phase progression (Fig. 1) (32).
We found that systemic 14-3-3ζ deletion in mice increased β-cell area (33); however, this increase in β-cell area could have been a compensatory response to decreased whole-body insulin sensitivity (18). Recently, we found that treatment of dispersed mouse and human islet preparations with pan-14-3-3 protein inhibitors significantly increased β-cell proliferation ex vivo, demonstrating a regulatory role of 14-3-3 proteins in β-cell proliferation (34). Moreover, we also reported that deletion of 14-3-3ζ in murine β-cells in vivo increased β-cell proliferation, as measured by the expression of proliferative markers (Ki-67 and PCNA), but surprisingly, this was not associated with a corresponding increase in β-cell mass (34). It is unclear why β-cell mass remained unchanged despite increased numbers of proliferating β-cells. One possibility is that 14-3-3ζ functions at specific checkpoints in the cell cycle and that other factors, including other 14-3-3 protein isoforms, are still needed to facilitate mitosis in β-cells (28). Additional studies are required to explore this further, as this would increase our understanding of the mechanisms that control β-cell proliferation.
Relationship Between 14-3-3 Proteins and DYRK1A-Mediated Proliferation
A proposed approach to treat type 1 and type 2 diabetes (T1D and T2D, respectively) is to induce the proliferation of β-cells to increase functional β-cell mass; however, whether this can be applied to the clinical setting is unclear. In humans, β-cell proliferation primarily occurs during the first few years of life, starting around birth and reaching its highest rate at the first postnatal year before drastically declining during early childhood (35). Adult human β-cells are generally refractory to proliferative stimuli and fail to undergo cell cycle progression and proliferation, at least in part due to a failure of cyclins and CDKs to undergo nuclear translocation (35,36).
Among identified regulators of β-cell proliferation, DYRK1A represents an intermediate that may link 14-3-3 protein activity with cell cycle progression in β-cells. DYRK1A modulates a broad range of biological functions, including mRNA splicing, apoptosis, cell division, and differentiation (37). DYRK1A inhibitors, such as harmine, promote murine and human β-cell proliferation in vitro and improve glucose homeostasis when administered to diabetic mice (37–39). Mechanistically, DYRK1A inhibition decreases CDKN1B/p27Kip1 stability, which permits cell cycle entry of human β-cells when assessed in vitro or in human islet transplantation studies (38). Direct interactions between 14-3-3 proteins and DYRK1A were discovered by yeast two-hybrid screening and coimmunoprecipitation approaches, and disrupting interactions between 14-3-3 proteins and DYRK1A inhibits DYRK1A kinase activity (40). Lastly, 14-3-3β has also been shown to interact with DYRK1A via phosphorylated Ser520, thereby increasing its kinase activity (Fig. 1) (41). Given the established role of DYRK1A in β-cell proliferation, these observations highlight the need to further explore the relationship between DYRK1A and 14-3-3 proteins in β-cells, especially in the context of proliferation.
Roles of 14-3-3 Proteins in Hormone Secretion
Glucose-stimulated insulin secretion (GSIS) from pancreatic β-cells occurs in response to elevated glycemia and is characterized by glucose entry, closure of KATP channels, opening of voltage-gated Ca2+ channels, and exocytosis of insulin-containing granules (Fig. 2A) (42). One of the first studies that implicated 14-3-3 proteins in the control of Ca2+-dependent hormone secretion was performed in adrenal chromaffin cells (43). Morgan and Burgoyne (43) identified a 30-kDa cytosolic protein, previously identified as EXO1, that potentiated protein kinase C–mediated Ca2+-dependent release of catecholamines. It was later determined that EXO1 shared sequence homology with 14-3-3 proteins.
We recently discovered a novel role for 14-3-3ζ and its related isoforms in the regulation of ATP-dependent insulin secretion (Fig. 2A). β-Cell-specific deletion of 14-3-3ζ in mice, as well as in vitro treatment of mouse and human islets with pan–14-3-3 protein inhibitors, enhanced GSIS without negatively impacting cell survival or growth. This was due to increased mitochondrial function, potentiated ATP synthesis, and upregulated expression of genes involved in oxidative phosphorylation (OXPHOS) (34). Moreover, in human islets from donors with T2D and isolated islets from db/db mice, pan-inhibition of 14-3-3 proteins also significantly potentiated GSIS, mitochondrial function, and ATP synthesis (34). Collectively, these observations demonstrate that 14-3-3ζ and its related isoforms restrain the full secretory potential of β-cells (Fig. 2A). As 14-3-3 proteins are known regulators of ATP synthase activity in mitochondria and chloroplasts in plants, a similar role of 14-3-3ζ may exist in mammalian cells, including pancreatic β-cells (44). Indeed, we have previously detected several ATP synthase subunits in the interactome of 14-3-3ζ in differentiating preadipocytes (25), and this warrants further studies to examine the relationship between 14-3-3ζ and ATP synthase complex formation and function in β-cells in the context of insulin secretion.
14-3-3ζ, Major Hub for the Regulation of Apoptosis and Cell Survival
One of the initial functions assigned to 14-3-3 proteins was the ability to regulate apoptosis (45), as it was shown that an inactive mutant of 14-3-3ζ markedly increased p38 mitogen-activated protein kinase–dependent apoptosis (45). The intrinsic apoptotic pathway is mediated by the BCL2 antagonist of cell death, BAD, and all seven mammalian isoforms of 14-3-3 proteins interact with BAD to promote cell survival (Fig. 1) (46). Human BAD binds to 14-3-3 proteins in a phosphorylation-dependent manner, with Ser74 and Ser75 serving as noncanonical and canonical 14-3-3 binding sites, respectively (47). In murine BAD, phosphorylated Ser112, Ser136, and Ser155 constitute canonical binding sites for 14-3-3 proteins (48). When apoptosis is initiated, dephosphorylation of BAD promotes its dissociation from 14-3-3 proteins and permits BAD translocation to mitochondria to inhibit the antiapoptotic BCL2-related protein long isoform (BCL-XL) (49). Another major effector of apoptosis, BCL2-associated X protein (BAX), is sequestered in the cytosol by 14-3-3θ in resting cells, and proapoptotic stimuli can promote caspase-dependent and -independent cleavage of 14-3-3θ to promote BAX mitochondrial translocation and the release of cytochrome c (Fig. 1) (50). We previously reported that 14-3-3ζ regulates MIN6 insulinoma cell survival, as its overexpression and depletion were associated with increased cytoplasmic sequestration of BAD and decreased cytoplasmic sequestration of BAX (51).
Taken together, these findings demonstrate important roles of 14-3-3 proteins in the regulation of cell survival, proliferation, and exocytosis, all of which are critical for optimal pancreatic β-cell function and glucose homeostasis.
How Does 14-3-3ζ Fit Into Alternative Metabolic Pathways That Regulate β-Cell Function?
The recent discovery of various alternative pathways that regulate insulin secretion has changed our perspective on how insulin release from the β-cell is controlled. Whether 14-3-3 proteins are involved in these pathways is not known, and below we discuss whether 14-3-3 proteins contribute to these pathways as well as newly evidenced cellular cross talk within pancreatic islets.
The Good Part of BAD
Although BAD is an established regulator of apoptosis, a series of seminal studies have also demonstrated its metabolic activity in mitochondria (52–54). Indeed, an enzymatic complex consisting of phosphorylated BAD (Ser112, Ser136, and Ser155) and glucokinase has been identified (52,53), whereby phosphorylated BAD is required for stabilizing the enzymatic complex and promoting optimal glucokinase-dependent mitochondrial respiration (54). Whole-body deletion of BAD in vivo, as well as transgenic overexpression of a nonphosphorylatable BAD mutant (Ser112/136/155A), resulted in impaired glucose tolerance in mice (54). Further examination of systemic BAD knockout mice and isolated islets revealed defective GSIS and impaired expansion of β-cell mass in response to high-fat diet feeding (52).
Of the three 14-3-3 protein binding sites in murine BAD, Ser112 and Ser136 are essential in the regulation of apoptosis (52–54). To date, the contributions of Ser155 to β-cell survival are not well-established, but phosphorylation of Ser155 in the BH3 domain of BAD has been found to shift the function of BAD from regulating apoptosis toward a metabolic role by promoting the enzymatic activity of glucokinase in mitochondria as well as positively influencing ATP-dependent GSIS. When phosphorylated BAD BH3 domain mimetics were administered in vitro and in vivo, β-cells were protected from inflammation-, hypoxia-, and nitric oxide–related apoptosis, along with enhanced GSIS, in a glucokinase-dependent manner. Moreover, these phosphorylated BAD BH3 domain mimetics were also able to prolong the survival and enhance the function of healthy mouse islets transplanted into diabetic, streptozotocin-treated mice to restore glucose homeostasis (53).
Given our finding that 14-3-3ζ has important roles in regulating insulin secretion, 14-3-3ζ may have dual roles in regulating ATP synthase activity (34) while also influencing the mitochondrial BAD-glucokinase complex, depending on the bioenergetic status of the β-cell. Depending on the phosphorylation status of BAD, 14-3-3ζ may have a critical role in balancing BAD’s apoptotic functions by limiting discrete pools of BAD to translocate to mitochondria to participate in the BAD-glucokinase complex (52,53) while sequestering the majority of BAD in the cytoplasm to prevent apoptosis (51). This suggests a novel area of study to further increase our understanding of GSIS and its regulation by BAD.
Is There Any Place for 14-3-3ζ in the New Mitocat-Mitoox Model?
A novel paradigm that challenges the canonical model of GSIS has been proposed, but whether 14-3-3ζ and its related isoforms are involved is unclear (55–57). According to this model, termed Mitocat-Mitoox, activation of membrane-associated pyruvate kinase (PK) leads to a localized increase in the ATP-to-ADP ratio to promote KATP channel closure, the subsequent rise in intracellular Ca2+, and insulin secretion (55–57). Energy consumption during this secretory process increases levels of ADP, which serves as a substrate for OXPHOS-fueled mitochondrial ATP synthesis necessary for sustained KATP channel closure and insulin secretion. It has been proposed that this cycle underlies glucose-induced oscillatory release of insulin (55–57). Given the scaffolding functions of 14-3-3ζ and its ability to compartmentalize proteins in a cell (51,58), it would be interesting to assess if, where, and how it regulates the activities and localization of key effectors of the Mitocat-Mitoox model. Moreover, how our recent finding of 14-3-3ζ–dependent regulation of ATP synthesis fits into this model will be of great interest to those who study insulin secretion. Collectively, these studies would increase our understanding of the pathways that facilitate GSIS.
Contributions of 14-3-3ζ in the Regulation of GSIS via Islet Cells Cross Talk
The α-cell has long been established as a cell type that is critically involved in the counterregulatory response to hypoglycemia, as it secretes glucagon to stimulate hepatic glucose output. However, it is increasingly evident that α-cells play an important role in the potentiation of GSIS. Accumulating evidence demonstrates that the proglucagon-derived peptides, glucagon and glucagon-like peptide-1 (GLP-1), contribute to GSIS (59,60). α-Cells exhibit heterogeneity in their hormone profile. While they typically produce glucagon, under certain circumstances, such as obesity and diabetes, some α-cells produce active GLP-1 (59,61). The regulation of this heterogeneity in α-cell hormone production is poorly understood.
We have found that enhanced β-cell GLP-1 receptor (GLP-1R) signaling, via surgical intervention or pharmacological activation, activates α-cell GLP-1 production in mouse islets both in vivo and in vitro and in human islets in vitro (60,62,63). We do not find an effect of GLP-1R agonist treatment of cultured α-TC1–6-cells to induce α-cell GLP-1 production, which is consistent with the literature reporting that, at most, only a small population of α-cells expresses the GLP-1R (64). Instead, the effect of β-cell GLP-1R signaling to activate α-cell GLP-1 production is mediated by a paracrine protein factor (60). While we have not yet identified the secreted factor that mediates this effect, our efforts to do so have identified downregulation of β-cell 14-3-3ζ expression as a key downstream regulator of this pathway (Fig. 2B) (60). Specifically, we found that GLP-1R activation downregulates β-cell 14-3-3ζ expression in human islets in vitro and mouse islets in vivo, but it does not do so in mouse islets with the β-cell GLP-1R knockout. Further, addition of a pan-inhibitor of 14-3-3 proteins increased active GLP-1 production and secretion in both mouse and human islets in vitro. Finally, addition of recombinant 14-3-3ζ ablated the ability of a GLP-1R agonist to activate mediators of α-cell GLP-1 production in mouse islets in vitro. Future work is needed to define the β-cell GLP-1R signaling cascade that regulates 14-3-3ζ expression and the proteins that interact with 14-3-3ζ within the β-cell that may become disinhibited in response to 14-3-3ζ downregulation. Such studies are of importance, as they will increase our understanding of how 14-3-3ζ mediates intraislet cross talk and how it acts in a paracrine manner to activate α-cell GLP-1 production.
These observations align with our previous finding where whole-body 14-3-3ζ ablation improved glucose tolerance in a GLP-1R–dependent manner (Fig. 2B) (33). In a similar vein, three 14-3-3 isoforms, β, ε, and θ, have also been associated with the protective effects of GLP-1R and glucokinase pathways against β-cell toxicity (65). Together, these findings provide compelling evidence that 14-3-3ζ regulates cross talk between β-cells and α-cells and supports a role for β-cell 14-3-3ζ as a negative regulator of GSIS in human and mouse islets (Fig. 2B).
“14-3-3”: A Secret Code to Enhance β-Cell Function and Proliferation?
One of the main goals in developing treatments for diabetes is to safely increase functional β-cell mass. Our findings that 14-3-3ζ has critical roles in the regulation of β-cell survival and function (33,34,51,60) raise the question of whether 14-3-3ζ represents a novel therapeutic target for the treatment of diabetes and other metabolic diseases.
Changes in the expression profiles of 14-3-3 protein isoforms have been confirmed in the context of T2D, which suggests that altered expression influences β-cell function. For example, recent single-cell RNA sequencing–based analyses of isolated islets from human subjects with T2D or mice exposed to cellular stresses in vitro showed that most 14-3-3 isoforms are upregulated in β-cells (66,67). We have also confirmed in samples of pancreatic islets from T2D human donors and db/db mice that Ywhaz/YWHAZ mRNA levels were increased compared with those of healthy, nondiabetic control samples (34). Further support for this notion was our finding that overexpression of 14-3-3ζ in mouse islets impaired β-cell function in vitro and in vivo (33,34). Of special interest was the discovery of single nucleotide polymorphisms associated with YWHAG, the gene coding for 14-3-3γ, that contribute to increased risk of islet dysfunction associated with T2D (68). Collectively, these observations suggest potential pathogenic effects of elevated expression of 14-3-3ζ and its related isoforms in β-cells.
To date, there are no approved therapies that have been specifically designed or developed to directly target 14-3-3ζ or its related isoforms. Fingolimod (FTY720) was initially developed as a treatment for multiple sclerosis and acts by decreasing the activity of the sphingosine-1-phosphate receptor, S1PR1 (69), and it has been shown to inactivate all 14-3-3 proteins by promoting their phosphorylation and causing the dissociation of 14-3-3 protein dimers (9). While it is unclear if fingolimod’s actions are due to inhibition of 14-3-3 proteins, administration of Fingolimod has beneficial effects on multiple aspects of glucose homeostasis and β-cell function and survival. For example, prolonged administration of fingolimod to diabetic db/db mice restores normoglycemia, glucose tolerance, and insulin sensitivity, in addition to promoting the growth and proliferation of pancreatic β-cells (70). These beneficial effects of fingolimod on β-cell function and proliferation are also maintained in spontaneously diabetic nonhuman primates (71). As an immunosuppressant, fingolimod protects grafted organs, including pancreatic islets, against autoimmune-mediated rejection by suppressing the infiltration of peripheral cytotoxic CD8+ T cells without affecting systemic immunity (72–75). Lastly, fingolimod is not reported to have detrimental effects on human islet function (76).
In-depth studies will be required to determine if it is possible to develop fingolimod-based approaches to target 14-3-3ζ and its related isoforms, which we have established are critical for β-cell function, proliferation, and survival (33,34,51,60). The ubiquitous expression of 14-3-3 proteins highlights the complexity of specifically targeting β-cells, and this potentially can be circumvented by elucidating the interactomes of 14-3-3ζ and its related isoforms in specific contexts, such as β-cell proliferation or GSIS (Fig. 3). This would permit the identification of specific mediators that function downstream of 14-3-3ζ in β-cells and that may represent alternative targets for the treatment of diabetes and obesity (Fig. 3). Additional studies in this domain are required before targeting 14-3-3ζ for the treatment of diabetes can come to fruition.
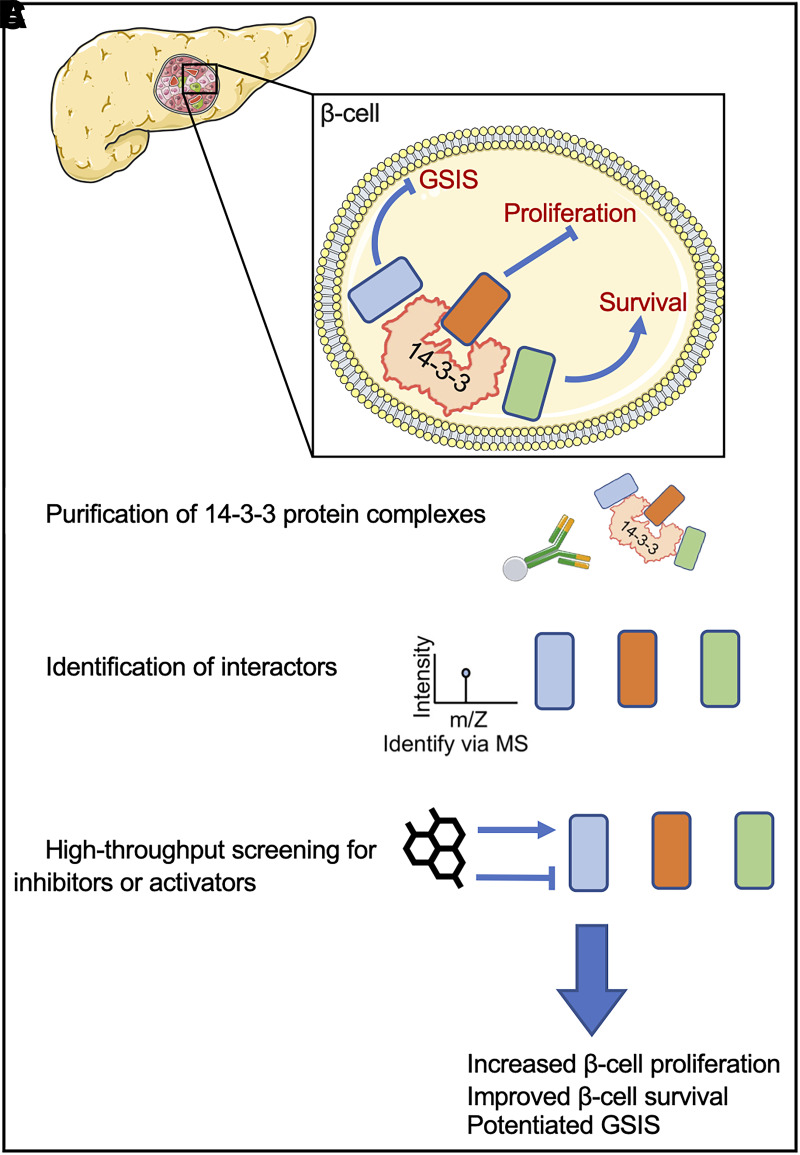
Figure 3.
Is the 14-3-3 protein interactome a pharmacological gold mine for protecting β-cells? 14-3-3ζ can interact with proteins harboring specific binding motifs created by phosphorylated serine (Ser) or threonine (Thr) residues, and this results in a dynamic interactome that can be elucidated for identifying novel regulators of β-cell function, proliferation, or survival. For example, using cadaveric human islets from healthy donors or those from donors with type 2 diabetes, 14-3-3ζ–anchored protein complexes can be purified (A) and subjected to mass spectrometry (MS) (B) to elucidate the interactome of 14-3-3ζ, and the contributions of identified interactors could then be determined by high-throughput screening assays or genomic approaches to assess their roles in β-cell survival, proliferation, and insulin secretion (C).
Conclusion
Although they were initially described as regulators of apoptosis and neurotransmitter synthesis and secretion, members of the 14-3-3 protein family have now been recognized as crucial regulators of metabolic signaling pathways. Moreover, their associations with the pathogenesis of chronic metabolic diseases, including diabetes and obesity, have become apparent. Among the seven mammalian 14-3-3 protein isoforms, we have identified 14-3-3ζ as a critical regulator of cell growth, proliferation, and apoptosis; glucose and lipid homeostasis; and islet and β-cell function. These ascribed functions of 14-3-3ζ are of particular interest, as they highlight the therapeutic potential of targeting 14-3-3ζ to treat metabolic diseases. In the context of diabetes, this could lead to the restoration of functional β-cell mass, which is a key determinant in the pathogenesis of both predominant forms of diabetes; however, it is important to note that this notion is very much in its infancy, and a large body of work will be required to comprehensively evaluate this possibility.
Article Information
Acknowledgments. The authors thank Servier Medical Art creators for allowing free access to their images, which were used to construct Figs. 1–3.
Funding. This work was funded by a National Science and Engineering Research Council Discovery Grant (RPGIN-2017-05209) and Canadian Institutes of Health Research Project Grants (PJT-186121 and PJT-432626) to G.E.L. and grants from the Department of Defense (W81XWH-18-1-0206), the Hartwell Foundation, and the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R56DK124853) to B.P.C. G.E.L. holds a Tier 2 Canada Research Chair in Adipocyte Development.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1. Jiang W, Tong T, Li W, et al. Molecular evolution of plant 14-3-3 proteins and function of Hv14-3-3A in stomatal regulation and drought tolerance. Plant Cell Physiol 2022;63:1857–1872 [DOI] [PubMed] [Google Scholar]
- 2. Zhang K, Huang Y, Shi Q. Genome-wide identification and characterization of 14-3-3 genes in fishes. Gene 2021;791:145721. [DOI] [PubMed] [Google Scholar]
- 3. Paul AL, Liu L, McClung S, Laughner B, Chen S, Ferl RJ. Comparative interactomics: analysis of Arabidopsis 14-3-3 complexes reveals highly conserved 14-3-3 interactions between humans and plants. J Proteome Res 2009;8:1913–1924 [DOI] [PubMed] [Google Scholar]
- 4. Abdrabou A, Brandwein D, Wang Z. Differential subcellular distribution and translocation of seven 14-3-3 isoforms in response to EGF and during the cell cycle. Int J Mol Sci 2020;21:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yaffe MB, Rittinger K, Volinia S, et al. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 1997;91:961–971 [DOI] [PubMed] [Google Scholar]
- 6. Waterman MJF, Stavridi ES, Waterman JLF, Halazonetis TD. ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat Genet 1998;19:175–178 [DOI] [PubMed] [Google Scholar]
- 7. Toleman CA, Schumacher MA, Yu SH, et al. Structural basis of O-GlcNAc recognition by mammalian 14-3-3 proteins. Proc Natl Acad Sci U S A 2018;115:5956–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsuruta F, Sunayama J, Mori Y, et al. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J 2004;23:1889–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodcock JM, Ma Y, Coolen C, et al. Sphingosine and FTY720 directly bind pro-survival 14-3-3 proteins to regulate their function. Cell Signal 2010;22:1291–1299 [DOI] [PubMed] [Google Scholar]
- 10. Woodcock JM, Coolen C, Goodwin KL, et al. Destabilisation of dimeric 14-3-3 proteins as a novel approach to anti-cancer therapeutics. Oncotarget 2015;6:14522–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Urano T, Saito T, Tsukui T, et al. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumour growth. Nature 2002;417:871–875 [DOI] [PubMed] [Google Scholar]
- 12. Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab 2011;13:68–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Q, Quan C, Xie B, et al. GARNL1, a major RalGAP α subunit in skeletal muscle, regulates insulin-stimulated RalA activation and GLUT4 trafficking via interaction with 14-3-3 proteins. Cell Signal 2014;26:1636–1648 [DOI] [PubMed] [Google Scholar]
- 14. Frøsig C, Pehmøller C, Birk JB, Richter EA, Wojtaszewski JFP. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol 2010;588:4539–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Q, Xie B, Zhu S, et al. A Tbc1d1 Ser231Ala-knockin mutation partially impairs AICAR- but not exercise-induced muscle glucose uptake in mice. Diabetologia 2017;60:336–345 [DOI] [PubMed] [Google Scholar]
- 16. Ogihara T, Isobe T, Ichimura T, et al. 14-3-3 protein binds to insulin receptor substrate-1, one of the binding sites of which is in the phosphotyrosine binding domain. J Biol Chem 1997;272:25267–25274 [DOI] [PubMed] [Google Scholar]
- 17. Neukamm SS, Ott J, Dammeier S, et al. Phosphorylation of serine 1137/1138 of mouse insulin receptor substrate (IRS) 2 regulates cAMP-dependent binding to 14-3-3 proteins and IRS2 protein degradation. J Biol Chem 2013;288:16403–16415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim GE, Albrecht T, Piske M, et al. 14-3-3ζ coordinates adipogenesis of visceral fat. Nat Commun 2015;6:7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elbein SC, Kern PA, Rasouli N, Yao-Borengasser A, Sharma NK, Das SK. Global gene expression profiles of subcutaneous adipose and muscle from glucose-tolerant, insulin-sensitive, and insulin-resistant individuals matched for BMI. Diabetes 2011;60:1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oppong AK, Diallo K, Robillard Frayne I, Des Rosiers C, Lim GE. Reducing 14-3-3ζ expression influences adipocyte maturity and impairs function. Am J Physiol Endocrinol Metab 2020;319:E117–E132 [DOI] [PubMed] [Google Scholar]
- 21. Ahmadian M, Abbott MJ, Tang T, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab 2011;13:739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marvyn PM, Mardian EB, Bradley RMA, Marks KA, Duncan RE. Data on hepatic lipolysis, adipose triglyceride lipase, and hormone-sensitive lipase in fasted and non-fasted C57BL/6J female mice. Data Brief 2016;7:721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 2011;50:14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diallo K, Dussault S, Noll C, et al. 14-3-3ζ mediates an alternative, non-thermogenic mechanism in male mice to reduce heat loss and improve cold tolerance. Mol Metab 2020;41:101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mugabo Y, Sadeghi M, Fang NN, Mayor T, Lim GE. Elucidation of the 14-3-3ζ interactome reveals critical roles of RNA-splicing factors during adipogenesis. J Biol Chem 2018;293:6736–6750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes: rationale and implications of the β-cell-centric classification schema. Diabetes Care 2016;39:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leal-Esteban LC, Fajas L. Cell cycle regulators in cancer cell metabolism. Biochim Biophys Acta Mol Basis Dis 2020;1866:165715. [DOI] [PubMed] [Google Scholar]
- 28. Hermeking H, Benzinger A. 14-3-3 proteins in cell cycle regulation. Semin Cancer Biol 2006;16:183–192 [DOI] [PubMed] [Google Scholar]
- 29. Nguyen A, Rothman DM, Stehn J, Imperiali B, Yaffe MB. Caged phosphopeptides reveal a temporal role for 14-3-3 in G1 arrest and S-phase checkpoint function. Nat Biotechnol 2004;22:993–1000 [DOI] [PubMed] [Google Scholar]
- 30. Laronga C, Yang HY, Neal C, Lee MH. Association of the cyclin-dependent kinases and 14-3-3 sigma negatively regulates cell cycle progression. J Biol Chem 2000;275:23106–23112 [DOI] [PubMed] [Google Scholar]
- 31. Kosaka Y, Cieslik KA, Li L, et al. 14-3-3ε plays a role in cardiac ventricular compaction by regulating the cardiomyocyte cell cycle. Mol Cell Biol 2012;32:5089–5102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dar A, Wu D, Lee N, Shibata E, Dutta A. 14-3-3 proteins play a role in the cell cycle by shielding cdt2 from ubiquitin-mediated degradation. Mol Cell Biol 2014;34:4049–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lim GE, Piske M, Lulo JE, Ramshaw HS, Lopez AF, Johnson JD. Ywhaz/14-3-3ζ deletion improves glucose tolerance through a GLP-1-dependent mechanism. Endocrinology 2016;157:2649–2659 [DOI] [PubMed] [Google Scholar]
- 34. Mugabo Y, Zhao C, Tan JJ, et al. 14-3-3ζ constrains insulin secretion by regulating mitochondrial function in pancreatic β cells. JCI Insight 2022;7:e156378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang P, Fiaschi-Taesch NM, Vasavada RC, Scott DK, García-Ocaña A, Stewart AF. Diabetes mellitus—advances and challenges in human β-cell proliferation. Nat Rev Endocrinol 2015;11:201–212 [DOI] [PubMed] [Google Scholar]
- 36. Fiaschi-Taesch NM, Kleinberger JW, Salim FG, et al. Human pancreatic β-cell G1/S molecule cell cycle atlas. Diabetes 2013;62:2450–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu T, Wang Y, Wang J, Ren C, Chen H, Zhang J. DYRK1A inhibitors for disease therapy: current status and perspectives. Eur J Med Chem 2022;229:114062. [DOI] [PubMed] [Google Scholar]
- 38. Dirice E, Walpita D, Vetere A, et al. Inhibition of DYRK1A stimulates human β-cell proliferation. Diabetes 2016;65:1660–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang P, Alvarez-Perez JC, Felsenfeld DP, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med 2015;21:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim D, Won J, Shin DW, et al. Regulation of Dyrk1A kinase activity by 14-3-3. Biochem Biophys Res Commun 2004;323:499–504 [DOI] [PubMed] [Google Scholar]
- 41. Alvarez M, Altafaj X, Aranda S, de la Luna S. DYRK1A autophosphorylation on serine residue 520 modulates its kinase activity via 14-3-3 binding. Mol Biol Cell 2007;18:1167–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rorsman P, Renström E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003;46:1029–1045 [DOI] [PubMed] [Google Scholar]
- 43. Morgan A, Burgoyne RD. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature 1992;355:833–836 [DOI] [PubMed] [Google Scholar]
- 44. Bunney TD, van Walraven HS, de Boer AH. 14-3-3 protein is a regulator of the mitochondrial and chloroplast ATP synthase. Proc Natl Acad Sci U S A 2001;98:4249–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xing H, Zhang S, Weinheimer C, Kovacs A, Muslin AJ. 14-3-3 proteins block apoptosis and differentially regulate MAPK cascades. EMBO J 2000;19:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Subramanian RR, Masters SC, Zhang H, Fu H. Functional conservation of 14-3-3 isoforms in inhibiting bad-induced apoptosis. Exp Cell Res 2001;271:142–151 [DOI] [PubMed] [Google Scholar]
- 47. Sluchanko NN, Tugaeva KV, Gushchin I, Remeeva A, Kovalev K, Cooley RB. Crystal structure of human 14-3-3ζ complexed with the noncanonical phosphopeptide from proapoptotic BAD. Biochem Biophys Res Commun 2021;583:100–105 [DOI] [PubMed] [Google Scholar]
- 48. Chiang CW, Kanies C, Kim KW, et al. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol 2003;23:6350–6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Won J, Kim DY, La M, Kim D, Meadows GG, Joe CO. Cleavage of 14-3-3 protein by caspase-3 facilitates bad interaction with Bcl-x(L) during apoptosis. J Biol Chem 2003;278:19347–19351 [DOI] [PubMed] [Google Scholar]
- 50. Nomura M, Shimizu S, Sugiyama T, et al. 14-3-3 interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem 2003;278:2058–2065 [DOI] [PubMed] [Google Scholar]
- 51. Lim GE, Piske M, Johnson JD. 14-3-3 proteins are essential signalling hubs for beta cell survival. Diabetologia 2013;56:825–837 [DOI] [PubMed] [Google Scholar]
- 52. Danial NN, Walensky LD, Zhang C-Y, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med 2008;14:144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ljubicic S, Polak K, Fu A, et al. Phospho-BAD BH3 mimicry protects β cells and restores functional β cell mass in diabetes. Cell Rep 2015;10:497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Danial NN, Gramm CF, Scorrano L, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 2003;424:952–956 [DOI] [PubMed] [Google Scholar]
- 55. Lewandowski SL, Cardone RL, Foster HR, et al. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab 2020;32:736–750.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Merrins MJ, Corkey BE, Kibbey RG, Prentki M. Metabolic cycles and signals for insulin secretion. Cell Metab 2022;34:947–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ho T, Potapenko E, Davis DB, Merrins MJ. A plasma membrane-associated glycolytic metabolon is functionally coupled to KATP channels in pancreatic α and β cells from humans and mice. Cell Rep 2023;42:112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hermeking H, Lengauer C, Polyak K, et al. 14-3-3σ is a p53-regulated inhibitor of G2/M progression. Mol Cell 1997;1:3–11 [DOI] [PubMed] [Google Scholar]
- 59. Holter MM, Saikia M, Cummings BP. Alpha-cell paracrine signaling in the regulation of beta-cell insulin secretion. Front Endocrinol (Lausanne) 2022;13:934775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Holter MM, Phuong DJ, Lee I, Saikia M, Weikert L, Fountain S, et al. 14-3-3-zeta mediates GLP-1 receptor agonist action to alter alpha-cell proglucagon processing. Sci Adv 2022;8:eabn3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Campbell SA, Golec DP, Hubert M, et al. Human islets contain a subpopulation of glucagon-like peptide-1 secreting α cells that is increased in type 2 diabetes. Mol Metab 2020;39:101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saikia M, Holter MM, Donahue LR, et al. GLP-1 receptor signaling increases PCSK1 and β cell features in human α cells. JCI Insight 2021;6:e141851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garibay D, Lou J, Lee SA, et al. β Cell GLP-1R signaling alters α cell proglucagon processing after vertical sleeve gastrectomy in mice. Cell Rep 2018;23:967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ast J, Arvaniti A, Fine NHF, et al. Super-resolution microscopy compatible fluorescent probes reveal endogenous glucagon-like peptide-1 receptor distribution and dynamics. Nat Commun 2020;11:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim MK, Cho JH, Lee JJ, Cheong YH, Son MH, Lee KJ. Differential protective effects of exenatide, an agonist of GLP-1 receptor and piragliatin, a glucokinase activator in beta cell response to streptozotocin-induced and endoplasmic reticulum stresses. PLoS One 2013;8:e73340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bosi E, Marselli L, De Luca C, Suleiman M, Tesi M, Ibberson M, et al. Integration of single-cell datasets reveals novel transcriptomic signatures of β-cells in human type 2 diabetes. NAR Genom Bioinform 2020;2:lqaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stancill JS, Kasmani MY, Khatun A, Cui W, Corbett JA. Single-cell RNA sequencing of mouse islets exposed to proinflammatory cytokines. Life Sci Alliance 2021;4:e202000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fernández-Tajes J, Gaulton KJ, van de Bunt M, et al. Developing a network view of type 2 diabetes risk pathways through integration of genetic, genomic and functional data. Genome Med 2019;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 2021;398:1184–1194 [DOI] [PubMed] [Google Scholar]
- 70. Zhao Z, Choi J, Zhao C, Ma ZA. FTY720 normalizes hyperglycemia by stimulating β-cell in vivo regeneration in db/db mice through regulation of cyclin D3 and p57(KIP2). J Biol Chem 2012;287:5562–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang Y, Liu Y, Wei Q, et al. FTY720 treatment rejuvenates β-cell function and improves cardiac function in diabetic nonhuman primates (NHPs). Diabetes 2018;67(Supplement_1):1121-P [Google Scholar]
- 72. Maeda A, Goto M, Zhang J, et al. Immunosuppression with FTY720 and cyclosporine A inhibits rejection of adult porcine islet xenografts in rats. Transplantation 2003;75:1409–1414 [DOI] [PubMed] [Google Scholar]
- 73. Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol 2000;164:5761–5770 [DOI] [PubMed] [Google Scholar]
- 74. Brinkmann V, Pinschewer D, Chiba K, Feng L. FTY720: a novel transplantation drug that modulates lymphocyte traffic rather than activation. Trends Pharmacol Sci 2000;21:49–52 [DOI] [PubMed] [Google Scholar]
- 75. Brinkmann V, Pinschewer DD, Feng L, Chen S. FTY720: altered lymphocyte traffic results in allograft protection. Transplantation 2001;72:764–769 [DOI] [PubMed] [Google Scholar]
- 76. Truong W, Emamaullee JA, Merani S, Anderson CC, James Shapiro AM. Human islet function is not impaired by the sphingosine-1-phosphate receptor modulator FTY720. Am J Transplant 2007;7:2031–2038 [DOI] [PubMed] [Google Scholar]