Abstract
Background
Plasma amyloid-β (Aβ) (Aβ42, Aβ40, and Aβ42/Aβ40), biomarkers of the Alzheimer’s form of dementia, are under consideration for clinical use. The associations of these peptides with circulating proteins may identify novel plasma biomarkers of dementia and inform peripheral factors influencing the levels of these peptides.
Methods
We analyzed the association of these 3 plasma Aβ measures with 4638 circulating proteins among a subset of the participants of the Atherosclerosis Risk in Communities (ARIC) study (midlife: n = 1955; late life: n = 2082), related the Aβ-associated proteins with incident dementia in the overall ARIC cohort (midlife: n = 11,069, late life: n = 4110) with external replication in the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study (n = 4973), estimated the proportion of Aβ variance explained, and conducted enrichment analyses to characterize the proteins associated with the plasma Aβ peptides.
Results
At midlife, of the 296 Aβ-associated proteins, 8 were associated with incident dementia from midlife and late life in the ARIC study, and NPPB, IBSP, and THBS2 were replicated in the AGES–Reykjavik Study. At late life, of the 34 Aβ-associated proteins, none were associated with incident dementia at midlife, and kidney function explained 10%, 12%, and 0.2% of the variance of Aβ42, Aβ40, and Aβ42/Aβ40, respectively. Aβ42-associated proteins at midlife were found to be enriched in the liver, and those at late life were found to be enriched in the spleen.
Conclusions
This study identifies circulating proteins associated with plasma Aβ levels and incident dementia and informs peripheral factors associated with plasma Aβ levels.
Keywords: Amyloid beta, Dementia, IBSP, NPPB, Proteomics, THBS2
Plasma amyloid-β (Aβ) peptides (Aβ42, Aβ40, and Aβ42/Aβ40) are under consideration for clinical use as biomarkers of Alzheimer’s disease (AD), the most common form of dementia (1,2). While Aβ40 is the most abundant Aβ peptide, the accumulation of Aβ42 fibrils in the brain is an early hallmark of AD pathology (3). Lower plasma levels of Aβ42 and Aβ42/Aβ40 have been associated with a higher risk for AD or dementia (4,5), likely reflecting lower clearance of Aβ42 from the brain (6).
Aβ peptides are cleaved from the protein encoded by Aβ precursor protein, which is expressed in a wide range of tissues (7,8). The circulating proteome consists of proteins that are secreted as messengers, tissue leakage products, immunoglobulins, and transmembrane proteins that shed from cells (9). Recently, high-throughput platforms have enabled studies of the circulating proteome with some replicated results for markers of dementia, such as natriuretic peptide B (10,11). While the utility of these new circulating markers needs further validation, identifying circulating proteins associated with plasma Aβ may reveal novel risk markers of dementia and increase our knowledge of the peripheral factors associated with plasma Aβ.
We conducted a large-scale cross-sectional proteomic analysis of the 3 plasma Aβ measures using 4638 proteins at midlife and late life in a subset of the participants of the Atherosclerosis Risk in Communities (ARIC) study (n = ∼2000 of 15,792) (12). We assessed the association of the Aβ-associated proteins with incident dementia in the overall ARIC cohort followed by replication in the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study; quantified the proportion of Aβ variances explained by demographic factors, kidney function, and Aβ-associated proteins; and characterized Aβ-associated protein signals using enrichment analysis.
Methods and Materials
Study Population for the Association Analysis of Plasma Aβ
The ARIC study enrolled 15,792 middle-aged adults from 4 U.S. communities (Washington County, Maryland; Forsyth County, North Carolina; Minneapolis, Minnesota; and Jackson, Mississippi) in 1987–1989 (visit 1). Up to December 2017, 6 visits were completed. At visit 3 (1993–1995) and visit 5 (2011–2013), a subset of the participants (n = ∼2000) had plasma Aβ and circulating protein available. The ARIC study was approved by each study site’s institutional review board. All participants provided written informed consent.
The selection of the participants for measuring plasma Aβ was reported previously (5). Briefly, at visit 5 (late life, 2011–2013; n = 6538), a subset of the participants were selected for additional cognitive assessments (n = 3976). Of these, plasma Aβ measures were available among 2576 participants at midlife and 2570 participants at late life. After excluding participants with missing data and those in race-center too small for analysis, we included 1955 participants at midlife and 2082 participants in late life. The number of participants excluded in each category is reported in Figure S1 in Supplement 1 for midlife and Figure S2 in Supplement 1 for late life. The population characteristics of the participants included and excluded from the analysis in midlife and late life are reported in Table S1 in Supplement 2.
Measurement of Plasma Aβ, Circulating Proteins, and Primary Covariates
Plasma samples were collected using EDTA tubes and stored at −80 °C until assayed based on a standardized protocol (13). Plasma Aβ assays were performed by the Department of Molecular Pharmacology and Experimental Therapeutics at The Mayo Clinic in Jacksonville, Florida, using the INNO-BIA immunoassay (Innogenetics), which had coefficients of variation (CVs) ranging from 1.9% to 7.2% for Aβ42 and 3.0% to 7.3% for Aβ40 (14). Samples with undetectable values were imputed to the limit of detection (12 pg/mL for Aβ42 and 15 pg/mL for Aβ40) (5).
Methods for the assay of circulating proteins at midlife and late life have been reported previously (11). Briefly, relative concentration of 5284 proteins in relative florescence units were quantified from plasma samples using SomaScan version 4, a modified aptamer-based platform, by SomaLogic, Inc. (15,16). The quality control process of SomaLogic, Inc involved normalization of the quantification levels by plate and sample followed by the protein levels from a pool of healthy control subjects (16). The median CVs from blinded duplicates were 6.3% at midlife and 4.7% at late life. We excluded aptamers having evidence for unspecific binding (n = 552, Table S2 in Supplement 2), deprecated (n = 7), or CV >50% or a variance of <0.01 on the log scale at either midlife or late life (n = 87). We included 4638 aptamers that quantified 4448 unique proteins or protein complexes.
Among the variables used as covariates, age, sex, and race were self-reported. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation with calibrated and standardized serum creatinine (17,18). The ascertainment of other variables used in the reporting of demographics or sensitivity analysis are reported in Supplemental Methods in Supplement 1.
Cross-sectional Association Analysis of Plasma Aβ Measures and Circulating Proteins
We report the Spearman correlations between the Aβ measures. Population characteristics by median of the Aβ measures were compared using t test for nonskewed continuous variables, Kruskal-Wallis test for skewed continuous variables, and χ2 test for categorical variables.
Given the lack of absolute quantification of the protein levels and the fact that it is unknown whether the correspondence between the relative unit and protein abundance at visits 3 and 5 was the same, we conducted the association analysis at midlife and late life in separate regression models. Each protein was used as the outcome with each Aβ measure as the predictor of interest. To reduce skewedness, we applied log2 transformation to the Aβ measures. For the protein levels, inspecting the large number of proteins for appropriate transformation seemed impractical; therefore, we applied rank-based inverse normal transformation. In our primary analysis, we aimed to include variables that capture broad hidden systematic factors that may affect protein levels. In addition to age, sex, and race-center, we included eGFR, which represents the influence of renal excretion on protein levels, and 4 probabilistic estimation of expression residuals factors (19,20). A brief summary of the probabilistic estimation of expression residuals factor methods is reported in Supplemental Methods in Supplement 1.
The statistical significance level was determined using the Bonferroni method in combination with permutation test (iterations = 1 million), which were performed for proteins with linear regression p value < 1.078 × 10−5 (=0.05/4638, the number of proteins tested). We considered a permutation p value < 1.078 × 10−5 as significant. The use of permutation test was motivated by the lack of methods to assess the extent of p value inflation in proteomic analysis (21). The permutation test is based on the empirical null distribution from the data rather than a theoretical null distribution. For the significant Aβ-associated proteins, we also report their Spearman correlations with their associated plasma Aβ. Figure 1 presents an overview of the association and subsequent characterization analyses.
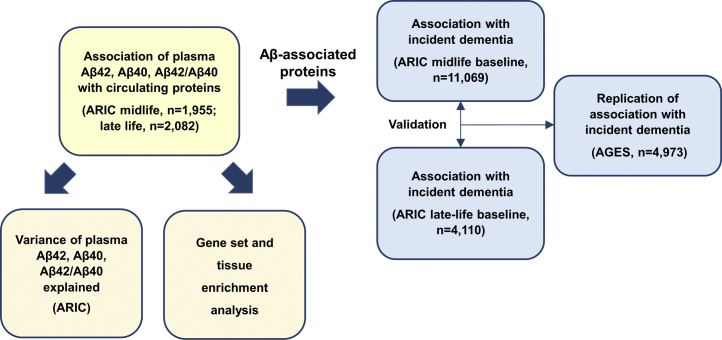
Figure 1.
Analysis flowchart. First, the analysis of the cross-sectional association between the plasma amyloid-β (Aβ) peptides (Aβ42, Aβ40, and Aβ42/Aβ40 ratio) and circulating protein was conducted in a subset of the Atherosclerosis Risk in Communities (ARIC) participants with plasma Aβ data available (yellow box). Next, circulating proteins with significant association with the Aβ peptides were assessed for their association with incident dementia in the overall ARIC cohort at two separated baselines followed by replication in the Age, Gene/Environment Susceptibility–Reykjavik Study (AGES) (blue boxes). Finally, the variances of the plasma Aβ peptides were quantified, and the results of the cross-sectional association between plasma Aβ and circulating proteins were further characterized using gene set and tissue enrichment analysis (orange boxes).
We conducted two sensitivity analyses: controlling for additional covariates related to chronic conditions and kidney function biomarkers. Details are reported in Supplemental Methods in Supplement 1. To assess whether the proteins that were associated with Aβ42 might be associated with the severity of cognitive impairment, we evaluated the association of these proteins with the scores of the Mini-Mental State Examination among those with dementia at late life. Details are reported in Supplemental Methods in Supplement 1.
Study Population for Incident Dementia Analysis
For proteins with significant association with one or more Aβ measures from permutation tests in the cross-sectional analysis, we evaluated their prospective associations with incident dementia in the overall ARIC cohort, where circulating proteins and incident dementia assessments were available in the overwhelming majority of the ARIC participants (n = 11,069 at midlife and 4110 at late life without prevalent dementia). The study population and methods used in this analysis have been reported (11). Incident dementia events were determined by an expert panel based on the criteria from the National Institute on Aging–Alzheimer’s Association workgroups and the DSM-5 or International Classification Disease codes at hospital discharge and on death certificates (22). Details of dementia ascertainment and participant inclusion criteria are reported in Supplemental Methods in Supplement 1.
Association Analysis of Plasma Aβ–Associated Proteins With Incident Dementia
To enhance the generalizability of the results, the association analysis of incident dementia used nonoverlapping cases from the midlife and late-life baselines. For the midlife baseline, participants were followed up to visit 5 (1131 incident dementia events). For the late-life baseline (visit 5, 2011–2013), participants were followed up to visit 6 (2016–2017, 428 incident dementia events). Participants who died or without detected dementia up to the end of the follow-up were censored.
A Cox regression model was evaluated controlling for age, sex, race-center, education levels, APOE ε4 carrier status, eGFR, and cardiovascular factors (current smoking status, body mass index, prevalent diabetes, and hypertension). We validated significant associations with incident dementia from midlife using the associations from late life and vice versa. The statistical significance levels were determined by the Bonferroni method (Table S3 in Supplement 2).
Replication of the Association of Aβ-Associated Proteins With Incident Dementia in the AGES–Reykjavik Study
The Aβ-associated proteins that were associated with incident dementia in ARIC (n = 8) were carried forward for replication for their association with incident dementia in AGES. Details of AGES and the AGES-Novartis SomaScan platform have been reported previously (23,24). Briefly, 5764 participants previously enrolled in the Reykjavik study were reexamined for the first wave of AGES in 2002–2006. AGES was approved by the Icelandic Nation Bioethics Committee (VSN 00-063), the Icelandic Data Protection Authority, Iceland, and the Institutional Review Board for the National Institute on Aging, National Institutes of Health, United States. All participants provided written informed consent.
Blood samples used for this study were collected at the first wave using a standardized protocol and stored at −80 °C. A total of 5034 SOMAmers were quantified. The interassay CVs determined using a subset of 1000 SOMAmers had a median of <1% (23).
Dementia classification was in accordance with DSM-IV criteria (25) and adjudicated based on consensus during conferences that included a neurologist, geriatrician, neuropsychologist, and neuroradiologist who provided a clinical reading of available magnetic resonance images. More details are provided in Supplemental Methods in Supplement 1.
Of the 5764 participants in AGES-Reykjavik study, 4973 participants without prevalent dementia and with data for covariates were included in the analyses. Participants were followed from June 2002 to October 2015. Dementia incidence rate was 27.5 (95% CI= 25.9–29.2) cases per 1000 person-years. A Cox regression model was used to examine the association between log2-transformed values of proteins and incident dementia controlling for baseline age, sex, education, APOE ε4 carrier status, eGFR, body mass index, diabetes, hypertension, and smoking status.
Evaluation of the Variance of Plasma Aβ Explained by Key Variables
To quantify the variability of the 3 plasma Aβ measures that could be explained by demographics (age, sex, and race), eGFR, and the Aβ-associated proteins, we evaluated 3 linear regression models using each Aβ measure as the dependent variable and obtained the R2 of these models, which can be interpreted as the proportion of the variance of the dependent variable explained by the independent variables. Model 1 included age, sex, and race-center. Model 2 added eGFR, and Model 3 added the Aβ-associated proteins based on permutation test.
Enrichment Analysis of Plasma Aβ–Associated Proteins
To characterize the protein association signals of the plasma Aβ measures, we conducted enrichment analyses using gene set enrichment analysis (GSEA) in the clusterProfiler package (version 3.16.1) (26). GSEA assesses whether members of a gene set tend to occur toward the top (or bottom) of the effect size distribution of the protein associations. The gene sets tested included those defined by Gene Ontology (GO) terms and pathways in the Kyoto Encyclopedia of Genes and Genomes and Reactome pathway databases (27, 28, 29). To summarize the enriched gene sets, we used a weighted set cover algorithm in the sumer package (version 1.1.5) to identify the smallest subset of the enriched gene sets that covers all genes in the enriched gene sets (30).
Tissue enrichment analysis was conducted using the TissueEnrich package version 1.10.1 and gene expression data of 53 human tissues from Genotype-Tissue Expression project version 8 after excluding Epstein–Barr virus–transformed lymphocytes (31). Based on a previous study on the human proteome, we defined tissue-enriched genes as those with at least 5 times higher gene expression in at least one tissue (32). The background gene list for tissue enrichment analysis consists of the intersection of the tissue-enriched genes and the encoding genes of all proteins tested. The input gene lists included the encoding genes of proteins associated with an Aβ measure at false discovery rate (FDR) < 0.01. p value was obtained using hypergeometric tests (33). We considered a gene set with an enrichment FDR < 0.05 and having at least 5 genes in the input list as significant.
Aggregate Association of Aβ-Associated Proteins in Enriched Gene Sets With Incident Dementia
For the proteins that were in GO terms significantly enriched for Aβ-association signals, we evaluated the aggregate association of these proteins with incident dementia. Enriched GO terms were separated into 3 groups: nervous system, immune system, and other (Table S4 in Supplement 2). We generated the first principal component (PC1) of the proteins to represent the overall variability using residuals of the protein levels after regressing out eGFR and the 4 probabilistic estimation of expression residuals factors. The association between PC1 and incident dementia was evaluated using the same study population and Cox regression model as in the analysis of incident dementia in ARIC reported above.
Results
Summary Statistics of the Study Population and the Aβ Measures
At midlife, the mean age was 59 years with 46% men (Table 1). At late life, the mean age was 77 years with 45% men. At both midlife and late life, the proportion of Black participants was 19% (Table S1 in Supplement 2). Participants with higher eGFR had lower Aβ42 or Aβ40 levels but similar Aβ42/Aβ40 levels (Tables S5 and S6 in Supplement 2). The correlations between the Aβ measures were moderate (absolute Spearman ρ ranged from 0.11 to 0.67) (Table S7 in Supplement 2).
Table 1.
Study Population Characteristics at Midlife (Visit 3) and Late Life (Visit 5)
| Variable | Midlife, n = 1955 | Late Life, n = 2082 |
|---|---|---|
| Age, Years, Mean (SD) | 59.28 (5.23) | 77.19 (5.29) |
| Male, n (%) | 891 (46%) | 930 (45%) |
| Black, n (%) | 369 (19%) | 403 (19%) |
| APOE ε4 Carrier, n (%) | 561 (30%) | 597 (30%) |
| ≥High School Education, n (%) | 1677 (86%) | 1788 (86%) |
| Body Mass Index, kg/m2, Mean (SD) | 28.26 (5.01) | 28.44 (5.58) |
| Hypertension, n (%) | 689 (35%) | 1533 (75%) |
| Diabetes, n (%) | 204 (10%) | 713 (35%) |
| Current Smoker, n (%) | 227 (12%) | 87 (5%) |
| Total Cholesterol, mg/dL, Mean (SD) | 208.38 (37.11) | 179.34 (41.42) |
| Prevalent Stroke, n (%) | 19 (1%) | 102 (5%) |
| Prevalent Heart Failure, n (%) | 54 (3%) | 300 (14%) |
| Prevalent Coronary Heart Disease, n (%) | 78 (4%) | 342 (17%) |
| eGFR, mL/min/1.73 m2, Mean (SD) | 88.44 (13.34) | 67.62 (17.27) |
| eGFR <60 mL/min/1.73 m2, n (%) | 37 (2%) | 678 (33%) |
| Aβ42, pg/mL, Mean (SD) | 29.65 (9.72) | 37.74 (11.59) |
| Aβ40, pg/mL, Mean (SD) | 166.15 (66.95) | 239.56 (87.32) |
| Aβ42/Aβ40, Mean (SD) | 0.20 (0.11) | 0.17 (0.08) |
Aβ, amyloid-β; eGFR, estimated glomerular filtration rate.
Cross-sectional Association of Plasma Aβ Measures With Circulating Proteins
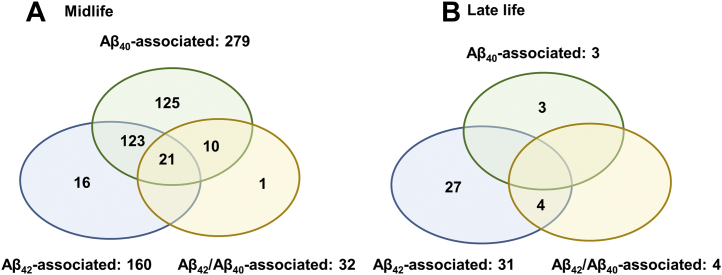
At midlife, 296 proteins had significant association with 1 or more plasma Aβ measures based on permutation test (160 for Aβ42, 279 for Aβ40, and 32 for Aβ42/Aβ40) (Figure 2A; Figure S3A–C in Supplement 1 and Table S8 in Supplement 2). At late life, 34 proteins had significant association with 1 or more plasma Aβ measures based on permutation test (31 for Aβ42, 3 for Aβ40, and 4 for Aβ42/Aβ40) (Figure 2B; Figure S4A–C in Supplement 1 and Table S9 in Supplement 2). Among the Aβ-associated proteins, only 5 were associated at both midlife and late life (Table S10 in Supplement 2). Table S11 in Supplement 2 reports the quality control information of the Aβ-associated proteins. Some of these Aβ-associated proteins have been linked to Aβ pathology, for example, clusterin and TREM2 (34, 35, 36). Some have been associated with incident dementia, for example, NPPB (11). Among the significant Aβ-associated proteins, their Spearman correlations with their respective Aβ measures were moderate (median = 0.14, first and third quartile = 0.06 and 0.26) (Tables S8 and S9 in Supplement 2; Figure S5 in Supplement 1).
Figure 2.
Number of circulating proteins that were significantly associated with levels of the plasma amyloid-β (Aβ) peptides (Aβ42 [blue], Aβ40 [green], Aβ42/Aβ40 ratio [orange]) at (A) midlife (n = 296) and (B) late life (n = 34).
In the sensitivity analysis controlling for 11 additional covariates, the sample size was 1834 at midlife and 1802 at late life. Of the 296 Aβ-associated proteins at midlife based on permutation test, 96% (n = 284) were associated with one or more Aβ measures at regression p value < 1.078 × 10−5 (Table S12 in Supplement 2). Of the 34 Aβ-associated proteins at late life, 24 were associated with one or more Aβ measures at late life at regression p value < 1.078 × 10−5 (Table S13 in Supplement 2). In the sensitivity analysis, controlling for additional biomarkers of kidney function, the associations were highly similar (Figures S6 and S7 in Supplement 1). Among 236 participants with dementia at late life, of the 31 Aβ42-associated proteins tested, complement C4A and C4B were associated with Mini-Mental State Examination at p = 1.45 × 10−2, and 74% (n = 23) had effect estimates consistent with their association with Aβ42 (Table S14 in Supplement 2).
Aβ-Associated Proteins and Incident Dementia in the ARIC Study and the AGES–Reykjavik Study
Next, to shed some light on whether the Aβ-associated proteins might be correlated with dementia pathogenesis, we evaluated the association of the Aβ-associated proteins with incident dementia in the overall ARIC cohort. At midlife, of the 296 Aβ-associated proteins, 33 were associated with incident dementia. Of these, 8 were also associated with incident dementia from the late-life baseline among nonoverlapping cases in ARIC (Table 2; Table S15 in Supplement 2). Higher levels of these 8 circulating proteins were associated with higher risk of incident dementia. For example, THBS2 was associated with a hazard ratio of 1.26 (95% CI = 1.14–1.40) at midlife and a hazard ratio of 1.29 (95% CI = 1.10–1.51) at late life. At late life, of the 34 Aβ-associated proteins, only C1QTNF3 was associated with incident dementia, and this association was not observed at midlife.
Table 2.
Association Between Aβ-Associated Proteins in Midlife and Incident Dementia
| UniProt Protein Name | Entrez Gene Name | Seq ID | ARIC Midlife |
ARIC Late Life |
AGES |
|||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |||
| Natriuretic Peptides Ba | NPPB | SeqId_7655_11 | 1.16 (1.09, 1.24) | 2.41 × 10−6 | 1.28 (1.18, 1.4) | 3.58 × 10−8 | 1.16 (1.08, 1.24) | 2.84 × 10−5 |
| Bone Sialoprotein 2a | IBSP | SeqId_3415_61 | 1.22 (1.12, 1.34) | 1.77 × 10−5 | 1.30 (1.14, 1.48) | 1.15 × 10−4 | 1.23 (1.08, 1.40) | .002 |
| Thrombospondin-2a | THBS2 | SeqId_3339_33 | 1.26 (1.14, 1.40) | 1.52 × 10−5 | 1.29 (1.10, 1.51) | 1.41 × 10−3 | 1.16 (1.05, 1.28) | .003 |
| RNA-Binding Protein EWS | EWSR1 | SeqId_12988_49 | 1.41 (1.21, 1.63) | 8.26 × 10−6 | 1.69 (1.32, 2.16) | 3.70 × 10−5 | 1.24 (1.03, 1.49) | .024 |
| Calsyntenin-3 | CLSTN3 | SeqId_6291_55 | 1.42 (1.27, 1.58) | 5.51× 10−10 | 1.38 (1.18, 1.62) | 7.01 × 10−5 | 1.18 (0.97, 1.42) | .092 |
| Protein ABHD14A | ABHD14A | SeqId_5715_4 | 1.45 (1.26, 1.67) | 2.44 × 10−7 | 1.48 (1.17, 1.87) | 9.83 × 10−4 | 1.15 (0.96, 1.36) | .122 |
| B Melanoma Antigen 2 | BAGE2 | SeqId_6294_11 | 1.43 (1.24, 1.65) | 1.11 × 10−6 | 1.73 (1.37, 2.19) | 5.32 × 10−6 | 1.15 (0.96, 1.38) | .127 |
| TNF Receptor Superfamily Member 1B | TNFRSF1B | SeqId_8368_102 | 1.48 (1.22, 1.79) | 7.33 × 10−5 | 1.56 (1.24, 1.95) | 1.24 × 10−4 | 1.15 (0.95, 1.39) | .139 |
Of the 296 proteins associated with at least one Aβ measure (Aβ42, Aβ40, Aβ42/Aβ40) based on permutation test at midlife (visit 3), 33 were associated with incident dementia with the midlife visit as the baseline (n = 11,069, incident dementia cases = 1131, p < 1.69 × 10−4 = 0.05/296). Of these 33 proteins, 8 were associated with incident dementia with the late-life visit as the baseline (visit 5, n = 4110, incident dementia cases = 428, p < 1.5 × 10−3 = 0.05/33). Replication in AGES was considered significant at p < .006 (=0.05/8). Hazard ratios were estimated per doubling of the protein levels in RFU.
Aβ, amyloid-β; AGES, Age, Gene/Environment Susceptibility–Reykjavik Study; ARIC, Atherosclerosis Risk in Communities Study; RFU, relative fluorescence unit.
Replicated in the AGES Study.
In the replication analysis in AGES (mean age = 76 years, 58% women) (Table S16 in Supplement 2), all 8 dementia-associated proteins from ARIC had a consistent direction of effect in AGES (p = .01). Three (NPPB, IBSP, and THBS2) were significantly associated with incident dementia in AGES after Bonferroni correction (p < 6.25 × 10−3 = 0.05/8) (Table 2; Table S17 in Supplement 2). The associations of IBSP and THBS2 were novel.
Variance of Plasma Aβ Measures Explained by Key Variables
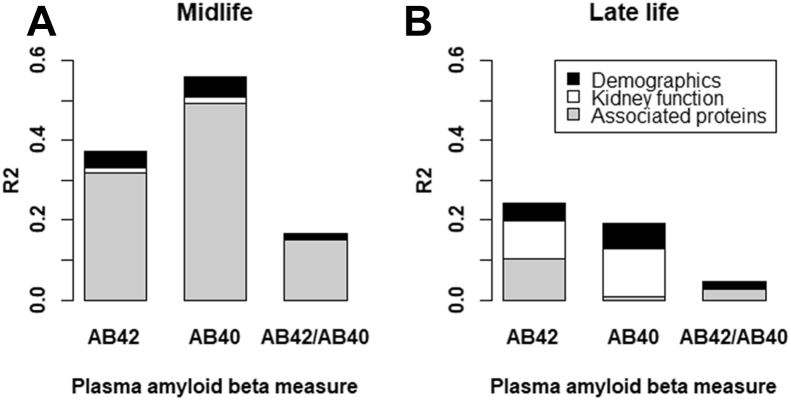
Demographic variables (age, sex, and race-center) explained similar variance for Aβ42 and Aβ40 at both midlife and late life (4.1%–6.4%) (Figure 3; Table S18 in Supplement 2). Kidney function explained 1.3% of the variances of Aβ42 and Aβ40 at midlife and 9.7% and 12.0%, respectively, at late life. The Aβ-associated proteins explained 31.9% and 49.4% of the variances of Aβ42 and Aβ40, respectively, at midlife and 10% or less at late life. The variance of Aβ42/Aβ40 ratio explained by demographic factors and kidney function was small (<2%).
Figure 3.
Variance of plasma amyloid β (AB) explained by demographic factors, kidney function, and associated proteins at midlife (A) and late life (B). Numerical values are reported in Table S18 in Supplement 2.
Gene Sets Enriched With the Encoding Genes of Aβ-Associated Proteins
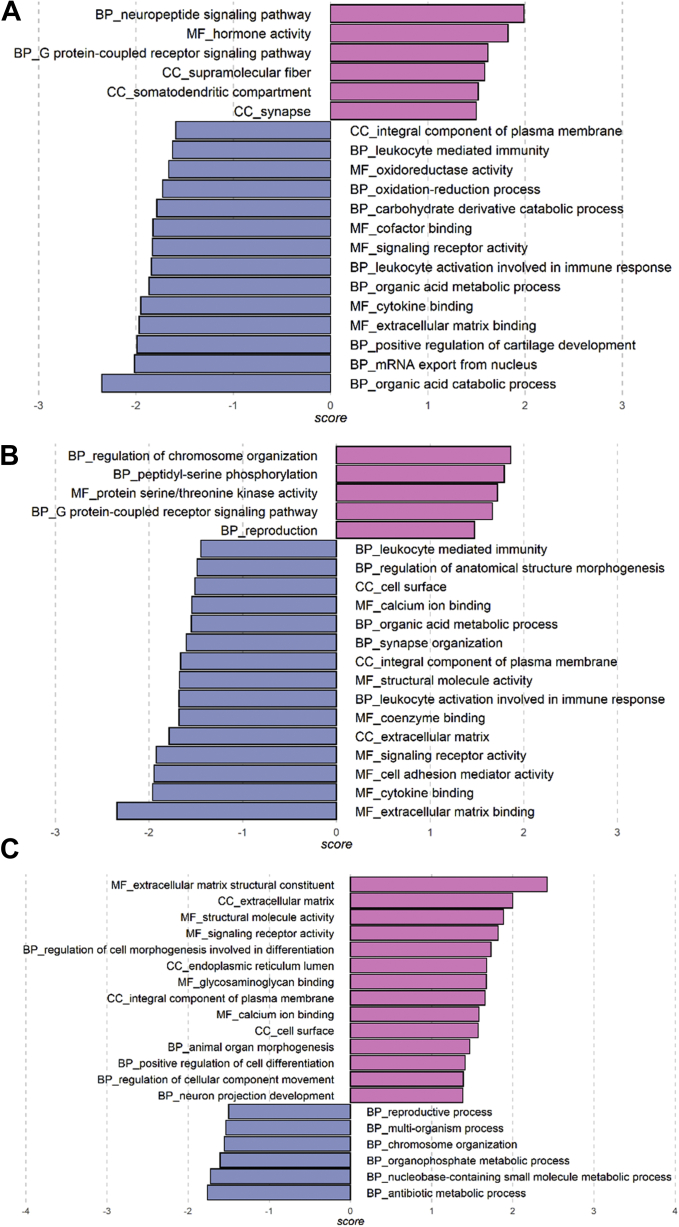
At midlife, GSEA detected enriched GO terms for all 3 sets of Aβ-associated signals, including GO terms related to the nervous system, e.g., neuropeptide signaling pathway, synapse organization, and neuron projection development (Figure 4A–C; Tables S19–S21 in Supplement 2). Seven GO terms related to general molecular function were common among the significant GO terms from the 3 sets of Aβ-associated signals (Table S22 in Supplement 2). At late life, the GO terms enriched for Aβ42-associated proteins were related to the immune system and general cellular processes (Figure S8 in Supplement 1 and Table S23 in Supplement 2). No GO terms were enriched for Aβ40- or Aβ42/Aβ40-associated protein signals. Among all the significant GO terms, only small molecule catabolic process was common between midlife and late life. No gene sets from Kyoto Encyclopedia of Genes and Genomes or Reactome pathways were significantly enriched in the midlife or late-life analyses.
Figure 4.
Gene Ontology terms significantly enriched for amyloid-β (Aβ)-associated protein signals at midlife based on gene set enrichment analysis. (A) Aβ42 associations. (B) Aβ40 associations. (C) Aβ42/Aβ40 associations. Details are reported in Tables S19–S21 in Supplement 2. BP, biological process; CC, cellular component; MF, molecular function.
In aggregate, the Aβ-associated proteins in the GO terms of the nervous system detected by GSEA were associated with incident dementia (PC1 of Aβ42-associated proteins p value 3.94 × 10−2; PC1 of Aβ40-associated protein p value 1.94 × 10−2) (Table S24 in Supplement 2). We annotated some proteins in these gene sets that have been linked to Aβ pathology by summarizing evidence from the literature (Table S25 in Supplement 2). Tissue enrichment analysis revealed that Aβ42-associated protein signals were enriched in gene expression in the liver at midlife (fold change 1.81, FDR 2.95 × 10−2) and in the spleen at late life (fold change 9.65, FDR 3.95 × 10−4). The enrichment in the liver was driven by genes involved in lipid metabolism and coagulation and in the spleen by genes related to antibody and antigen function (Table S26 in Supplement 2). No enriched tissues were detected for the Aβ40- or Aβ42/Aβ40-associated proteins at midlife or late life.
Discussion
In this large-scale proteomic analysis of circulating proteins, we identified associated proteins of plasma Aβ at midlife and late life. Some Aβ-associated proteins are emerging plasma biomarkers of dementia, including NPPB, clusterin, and TREM2 (11,34,36). Of the 3 Aβ-associated proteins with replicated evidence for their associations with incident dementia, two (THBS2 and IBSP) were novel. Demographic and peripheral factors, such as kidney function, explained a substantial proportion of the variance of Aβ42 and Aβ40 at late life. Our results extend our knowledge on the associated factors of plasma Aβ and circulating protein association with incident dementia.
While the clinical use of the plasma Aβ measures as biomarkers of cerebral amyloidosis is under consideration (2,4,37,38), studies on the peripheral factors associated with plasma Aβ are still limited (8,39). In our results, the Aβ-associated proteins in midlife and late life had few overlaps. A study on the associations between circulating proteins and cognitive function also reported different associations in midlife versus late life (40). These differences in associations between age groups may be a result of changes in circulating protein levels from midlife to late life (41). The enrichment of tissue gene expression at midlife and late life for the Aβ42-associated proteins is consistent with the important role of the liver in circulating protein production and lipid regulation and the spleen in immune function (42,43). These results suggest the large number of Aβ-associated proteins at midlife might be partly driven by liver function, which has been reported to influence Aβ clearance (44,45). The Aβ-associations during late life might be partly driven by immune response, which is an implicated pathway underlying AD and may be related to chronic inflammation in older age (46).
The proportions of the variance of plasma Aβ42 and Aβ40 explained by kidney function were substantial in late life. Recently, plasma Aβ42 levels were reported to be 1 standard deviation higher among older adults with chronic kidney disease than those without chronic kidney disease (47). Renal filtration and excretion have been implicated as an organ for the peripheral clearance of Aβ42 and Aβ40 (48). In contrast, the variability of plasma Aβ42/Aβ40 in both midlife and late life appeared to have little association with demographic factors and kidney function, which provides an advantage for plasma Aβ42/Aβ40 as a biomarker of AD. Our results suggest the need for large studies with diverse populations to evaluate the extent that performance characteristics of the tests using plasma Aβ might be affected by functions of peripheral organs.
The 3 replicated Aβ-associated proteins for incident dementia have been linked to vascular risk factors of dementia. NPPB, a hormone mainly expressed by heart muscle, is an established biomarker of heart failure and has been associated with stroke and atrial fibrillation, both risk factors of dementia (49, 50, 51). IBSP is a major structural protein of the bone matrix. Its encoding gene was found to be upregulated in stenotic human aortic valves and carotid plaques (52,53). THBS2 is believed to have a role in regulating angiogenesis. Its encoding gene has higher expression in stenotic aortic valves (54). Circulating THBS2 has been reported to be higher among patients with stroke (55). Aberrant angiogenesis has been linked to AD pathology (11,56,57).
Our study has several strengths. We identified a large number of circulating Aβ-associated proteins at midlife and late life. The associations of the Aβ-associated proteins with incident dementia were validated at two time points using nonoverlapping cases in ARIC with some protein associations replicated in AGES. Some limitations warrant mentioning. For characterizing the peripheral influence on Aβ levels, we focused on the kidney and did not have measures of liver function, an important determinant of circulating protein levels. The levels of the circulating proteins were relative measures, which limited our ability to assess changes in protein levels between midlife and late life. The Aβ assay was not as sensitive as the more recent assays and might have limited our ability to detect associations (37). As a cross-sectional association study of circulating proteins and the Aβ peptides, our results cannot infer a causal relationship between the Aβ-associated proteins and Aβ pathology in the brain. Studies using protein levels in cerebrospinal fluid would likely be more informative of AD pathologies. Our cross-sectional study cannot infer whether any chronic conditions, such as high body mass index, might be upstream factors or consequences of certain circulating protein levels. We did not have the relevant variables to accurately classify subtypes of dementia, such as AD and vascular dementia, to further characterize the association of the Aβ-associated proteins with dementia. Although the Aβ measures, particularly Aβ42/40, are established biomarkers of AD, these biomarkers are not surrogates for AD diagnosis.
In summary, this cross-sectional association analysis of the plasma Aβ peptides and circulating proteins identified a large number of Aβ-associated proteins in midlife and late life and extended our knowledge of the associated proteins of the plasma Aβ peptides and incident dementia.
Acknowledgments and Disclosures
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute (Grant Nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I [principal investigator, JC]). Neurocognitive data collection was funded by from the National Institutes of Health (National Heart, Lung, and Blood Institute, National Institute of Neurological Disorders and Stroke, National Institute on Aging (NIA), and National Institute on Deafness and Other Communication Disorders) (Grant Nos. U01 2U01HL096812 [principal investigator, JC], 2U01HL096814, 2U01HL096899, 2U01HL096902, and 2U01HL096917 [principal investigator, THM]), and previous brain magnetic resonance imaging examinations were funded by the National Heart, Lung, and Blood Institute (Grant No. R01-HL70825 [principal investigator, RG]). KAW was funded by the NIA Intramural Research Program. This research was funded, in part, by the NIA Intramural Research Program and the National Institute of Neurological Disorders and Stroke.
The AGES study is funded by the Icelandic Heart Association (Grant No. HHSN271201200022C), NIA (Grant No. N01-AG-12100), National Institutes of Health (Grant No. 1R01AG065596-01A1), and Althingi (the Icelandic Parliament). The AGES study was supported by the Novartis Institute for Biomedical Research, and protein measurements for the AGES cohort were performed at SomaLogic.
We thank authors the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
LLJ is an employee and stockholder of Novartis. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
AT and KJS contributed equally to this work.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.04.005.
Supplementary Material
References
- 1.(2021): 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 2.Shen X.N., Li J.Q., Wang H.F., Li H.Q., Huang Y.Y., Yang Y.X., et al. Plasma amyloid, tau, and neurodegeneration biomarker profiles predict Alzheimer’s disease pathology and clinical progression in older adults without dementia. Alzheimers Dement (Amst) 2020;12 doi: 10.1002/dad2.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack C.R., Jr., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S., et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chouraki V., Beiser A., Younkin L., Preis S.R., Weinstein G., Hansson O., et al. Plasma amyloid-β and risk of Alzheimer’s disease in the Framingham Heart Study. Alzheimers Dement. 2015;11:249–257.e1. doi: 10.1016/j.jalz.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan K.J., Blackshear C., Simino J., Tin A., Walker K.A., Sharrett A.R., et al. Association of midlife plasma amyloid-β levels with cognitive impairment in late life: The ARIC Neurocognitive Study. Neurology. 2021;97:e1123–e1131. doi: 10.1212/WNL.0000000000012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Gu B.J., Masters C.L., Wang Y.J. A systemic view of Alzheimer disease – Insights from amyloid-β metabolism beyond the brain [published correction appears in Nat Rev Neurol 2017; 13:703] Nat Rev Neurol. 2017;13:612–623. doi: 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- 7.Müller U.C., Deller T., Korte M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat Rev Neurosci. 2017;18:281–298. doi: 10.1038/nrn.2017.29. [DOI] [PubMed] [Google Scholar]
- 8.Roher A.E., Esh C.L., Kokjohn T.A., Castaño E.M., Van Vickle G.D., Kalback W.M., et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson N.L., Anderson N.G. The human plasma proteome: History, character, and diagnostic prospects [published correction appears in Mol Cell Proteomics 2003; 2:50] Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 10.Guo L.H., Alexopoulos P., Wagenpfeil S., Kurz A., Perneczky R., Alzheimer’s Disease Neuroimaging Initiative Plasma proteomics for the identification of Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27:337–342. doi: 10.1097/WAD.0b013e31827b60d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker K.A., Chen J., Zhang J., Fornage M., Yang Y., Zhou L., et al. Large-scale plasma proteomic analysis identifies proteins and pathways associated with dementia risk. Nat Aging. 2021;1:473–489. doi: 10.1038/s43587-021-00064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright J.D., Folsom A.R., Coresh J., Sharrett A.R., Couper D., Wagenknecht L.E., et al. The ARIC (Atherosclerosis Risk in Communities) study: JACC Focus Seminar 3/8. J Am Coll Cardiol. 2021;77:2939–2959. doi: 10.1016/j.jacc.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papp A.C., Hatzakis H., Bracey A., Wu K.K. ARIC hemostasis study--I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61:15–19. [PubMed] [Google Scholar]
- 14.Blennow K., De Meyer G., Hansson O., Minthon L., Wallin A., Zetterberg H., et al. Evolution of Abeta42 and Abeta40 levels and Abeta42/Abeta40 ratio in plasma during progression of Alzheimer’s disease: A multicenter assessment. J Nutr Health Aging. 2009;13:205–208. doi: 10.1007/s12603-009-0059-0. [DOI] [PubMed] [Google Scholar]
- 15.Gold L., Ayers D., Bertino J., Bock C., Bock A., Brody E.N., et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams S.A., Kivimaki M., Langenberg C., Hingorani A.D., Casas J.P., Bouchard C., et al. Plasma protein patterns as comprehensive indicators of health. Nat Med. 2019;25:1851–1857. doi: 10.1038/s41591-019-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 2011; 155:408] Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvin E., Manzi J., Stevens L.A., Van Lente F., Lacher D.A., Levey A.S., Coresh J. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Stegle O., Parts L., Durbin R., Winn J. A Bayesian framework to account for complex non-genetic factors in gene expression levels greatly increases power in eQTL studies. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Brody E.N., Murthy A.C., Mehler R.E., Weiss S.J., DeLisle R.K., et al. Impact of kidney function on the blood proteome and on protein cardiovascular risk biomarkers in patients with stable coronary heart disease. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manrai A.K., Ioannidis J.P.A., Patel C.J. Signals among signals: Prioritizing nongenetic associations in massive data sets. Am J Epidemiol. 2019;188:846–850. doi: 10.1093/aje/kwz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knopman D.S., Gottesman R.F., Sharrett A.R., Wruck L.M., Windham B.G., Coker L., et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) Alzheimers Dement (Amst) 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emilsson V., Ilkov M., Lamb J.R., Finkel N., Gudmundsson E.F., Pitts R., et al. Co-regulatory networks of human serum proteins link genetics to disease. Science. 2018;361:769–773. doi: 10.1126/science.aaq1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris T.B., Launer L.J., Eiriksdottir G., Kjartansson O., Jonsson P.V., Sigurdsson G., et al. Age, Gene/Environment Susceptibility-Reykjavik Study: Multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders 4th ed., Text Revision. Washington, DC: American Psychiatric Publishers.
- 26.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schriml L.M., Mitraka E., Munro J., Tauber B., Schor M., Nickle L., et al. Human Disease Ontology 2018 update: Classification, content and workflow expansion. Nucleic Acids Res. 2019;47:D955–D962. doi: 10.1093/nar/gky1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabregat A., Jupe S., Matthews L., Sidiropoulos K., Gillespie M., Garapati P., et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018;46:D649–D655. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savage S.R., Shi Z., Liao Y., Zhang B. Graph algorithms for condensing and consolidating gene set analysis results [published correction appears in Mol Cell Proteomics 2020; 19:431. Mol Cell Proteomics. 2019;18(suppl 1):S141–S152. doi: 10.1074/mcp.TIR118.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GTEx Consortium The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 33.Jain A., Tuteja G. TissueEnrich: Tissue-specific gene enrichment analysis. Bioinformatics. 2019;35:1966–1967. doi: 10.1093/bioinformatics/bty890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beeg M., Stravalaci M., Romeo M., Carrá A.D., Cagnotto A., Rossi A., et al. Clusterin binds to Aβ1-42 oligomers with high affinity and interferes with peptide aggregation by inhibiting primary and secondary nucleation. J Biol Chem. 2016;291:6958–6966. doi: 10.1074/jbc.M115.689539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson A.R., Sagare A.P., Zlokovic B.V. Role of clusterin in the brain vascular clearance of amyloid-β. Proc Natl Acad Sci U S A. 2017;114:8681–8682. doi: 10.1073/pnas.1711357114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kober D.L., Stuchell-Brereton M.D., Kluender C.E., Dean H.B., Strickland M.R., Steinberg D.F., et al. Functional insights from biophysical study of TREM2 interactions with apoE and Aβ1-42 [published online Oct 8] Alzheimers Dement. 2020 doi: 10.1002/alz.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmqvist S., Janelidze S., Stomrud E., Zetterberg H., Karl J., Zink K., et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related β-amyloid status. JAMA Neurol. 2019;76:1060–1069. doi: 10.1001/jamaneurol.2019.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindler S.E., Bollinger J.G., Ovod V., Mawuenyega K.G., Li Y., Gordon B.A., et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93:e1647–e1659. doi: 10.1212/WNL.0000000000008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janelidze S., Stomrud E., Palmqvist S., Zetterberg H., van Westen D., Jeromin A., et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep. 2016;6 doi: 10.1038/srep26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris S.E., Cox S.R., Bell S., Marioni R.E., Prins B.P., Pattie A., et al. Neurology-related protein biomarkers are associated with cognitive ability and brain volume in older age. Nat Commun. 2020;11:800. doi: 10.1038/s41467-019-14161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehallier B., Gate D., Schaum N., Nanasi T., Lee S.E., Yousef H., et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25:1843–1850. doi: 10.1038/s41591-019-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trefts E., Gannon M., Wasserman D.H. The liver. Curr Biol. 2017;27:R1147–R1151. doi: 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis S.M., Williams A., Eisenbarth S.C. Structure and function of the immune system in the spleen. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maarouf C.L., Walker J.E., Sue L.I., Dugger B.N., Beach T.G., Serrano G.E. Impaired hepatic amyloid-beta degradation in Alzheimer’s disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y.R., Wang Q.H., Zhang T., Liu Y.H., Yao X.Q., Zeng F., et al. Associations between hepatic functions and plasma amyloid-beta levels-Implications for the capacity of liver in peripheral amyloid-beta clearance. Mol Neurobiol. 2017;54:2338–2344. doi: 10.1007/s12035-016-9826-1. [DOI] [PubMed] [Google Scholar]
- 46.International Genomics of Alzheimer’s Disease Consortium (IGAP) Convergent genetic and expression data implicate immunity in Alzheimer’s disease. Alzheimers Dement. 2015;11:658–671. doi: 10.1016/j.jalz.2014.05.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Syrjanen J.A., Campbell M.R., Algeciras-Schimnich A., Vemuri P., Graff-Radford J., Machulda M.M., et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2022;18:1128–1140. doi: 10.1002/alz.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian D.Y., Cheng Y., Zhuang Z.Q., He C.Y., Pan Q.G., Tang M.Z., et al. Physiological clearance of amyloid-beta by the kidney and its therapeutic potential for Alzheimer’s disease. Mol Psychiatry. 2021;26:6074–6082. doi: 10.1038/s41380-021-01073-6. [DOI] [PubMed] [Google Scholar]
- 49.Goetze J.P., Bruneau B.G., Ramos H.R., Ogawa T., de Bold M.K., de Bold A.J. Cardiac natriuretic peptides. Nat Rev Cardiol. 2020;17:698–717. doi: 10.1038/s41569-020-0381-0. [DOI] [PubMed] [Google Scholar]
- 50.Chen L.Y., Norby F.L., Gottesman R.F., Mosley T.H., Soliman E.Z., Agarwal S.K., et al. Association of atrial fibrillation with cognitive decline and dementia over 20 years: The ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study) J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pendlebury S.T., Rothwell P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 52.Bossé Y., Miqdad A., Fournier D., Pépin A., Pibarot P., Mathieu P. Refining molecular pathways leading to calcific aortic valve stenosis by studying gene expression profile of normal and calcified stenotic human aortic valves. Circ Cardiovasc Genet. 2009;2:489–498. doi: 10.1161/CIRCGENETICS.108.820795. [DOI] [PubMed] [Google Scholar]
- 53.Ayari H., Bricca G. Microarray analysis reveals overexpression of IBSP in human carotid plaques. Adv Med Sci. 2012;57:334–340. doi: 10.2478/v10039-012-0056-0. [DOI] [PubMed] [Google Scholar]
- 54.Pohjolainen V., Mustonen E., Taskinen P., Näpänkangas J., Leskinen H., Ohukainen P., et al. Increased thrombospondin-2 in human fibrosclerotic and stenotic aortic valves. Atherosclerosis. 2012;220:66–71. doi: 10.1016/j.atherosclerosis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Navarro-Sobrino M., Rosell A., Hernández-Guillamon M., Penalba A., Boada C., Domingues-Montanari S., et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216:205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Vergara M.I., Rosales-Nieves A.E., March-Diaz R., Rodriguez-Perinan G., Lara-Ureña N., Ortega-de San Luis C., et al. Non-productive angiogenesis disassembles Aß plaque-associated blood vessels. Nat Commun. 2021;12:3098. doi: 10.1038/s41467-021-23337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh C.S.B., Choi K.B., Munro L., Wang H.Y., Pfeifer C.G., Jefferies W.A. Reversing pathology in a preclinical model of Alzheimer’s disease by hacking cerebrovascular neoangiogenesis with advanced cancer therapeutics. EBioMedicine. 2021;71 doi: 10.1016/j.ebiom.2021.103503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.