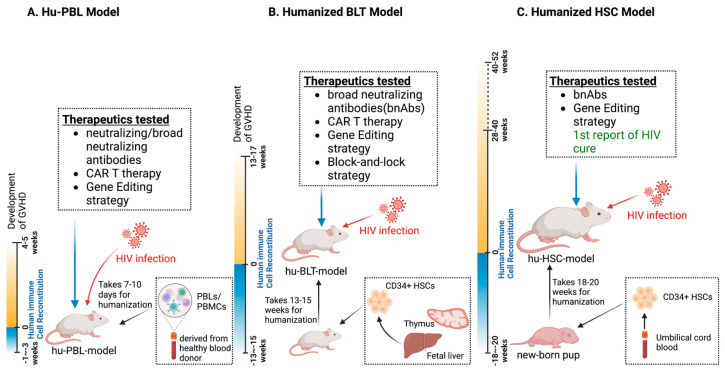
Figure 1.
Humanized mouse models for preclinical HIV therapeutics. (A) The hu-PBL model employs peripheral blood mononuclear cells (PBMC/PBLs) from human donors that are injected into immunocompromised mice. This model typically reaches functional maturity within 1–2 weeks but is highly susceptible to graft-versus-host disease (GVHD) within 4–5 weeks of cell implantation. The hu-PBL model is commonly used for drug discovery and quick screening of antiretroviral drug combinations as well as novel antiretroviral therapies. (B) The hu-BLT model involves the transplantation of human HSCs and fetal liver and thymus tissues into immunocompromised mice to generate a more complete human immune system. This model requires 13–15 weeks for human immune cells to reach full functional maturity, and it has been extensively used as a chronic HIV infection model for HIV latency and cure studies, enabling therapy testing for more than 10 weeks. (C) The hu-HSC model utilizes umbilical-cord-blood-isolated CD34+ hematopoietic stem cells (HSCs) that are transplanted into newborn pups to generate functional human immune systems. It necessitates 18–20 weeks for full maturation and allows for long-term testing of HIV reservoir establishment and therapeutic targeting studies for up to one year. The first report of an HIV cure in a humanized mouse model used sequential long-acting ART and CRISPR-based gene editing treatments. This model has better clinical translational potential than the other models.