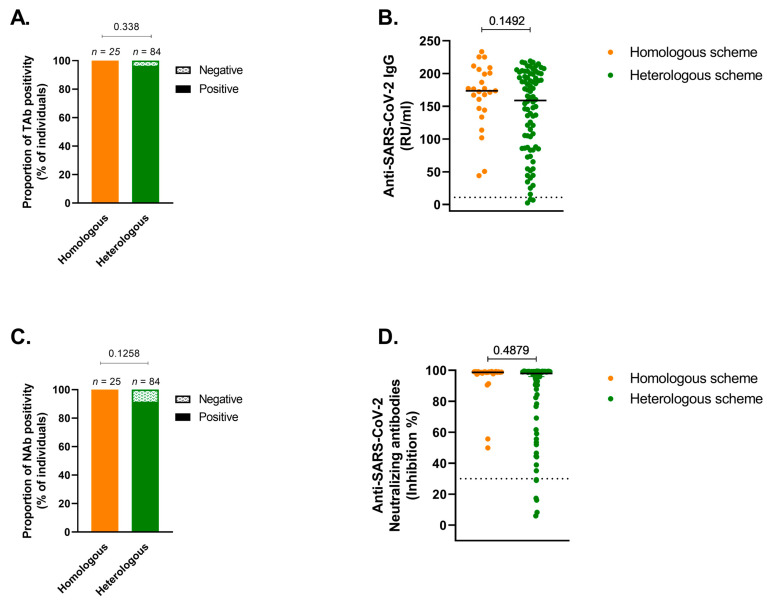
Figure 2.
Humoral response against SARS-CoV-2 among solid cancer patients receiving either a homologous or heterologous vaccination schedule. (A) proportion of total IgG (TAb) anti-S1 positivity (≥11 relative units per mL, RU/mL), (B) total IgG anti-S1 GMC (95%CI), RU/mL), (C) proportion of neutralizing antibody (NAb) positivity (≥30% of inhibition rate), and (D) neutralizing activity (median (IQR) of percentage of inhibition). Dotted lines in (B,D) indicate seropositivity cut-offs.