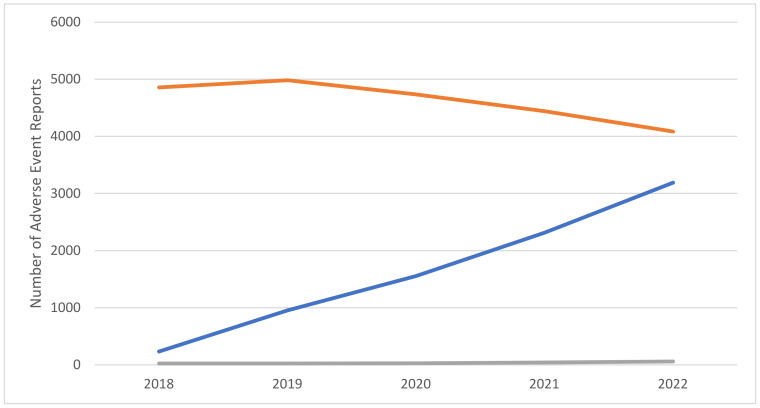
Figure 1.
Number of adverse event reports (AERs) involving semaglutide, other glucagon-like peptide-1 (GLP-1) analogues (dulaglutide, liraglutide, exenatide, lixisenatide, tirzepatide, and albiglutide), and the combination phentermine–topiramate. Data source: Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS; 2018–2022). Legend: semaglutide: blue color; other GLP-1 analogues: orange; phentermine–topiramate: grey.