Abstract
Hashimoto’s thyroiditis (HT) is a common autoimmune disease affecting the thyroid gland, characterized by lymphocytic infiltration, damage to thyroid cells, and hypothyroidism, and often requires lifetime treatment with levothyroxine. The disease has a complex etiology, with genetic and environmental factors contributing to its development. Vitamin D deficiency has been linked to a higher prevalence of thyroid autoimmunity in certain populations, including children, adolescents, and obese individuals. Moreover, vitamin D supplementation has shown promise in reducing antithyroid antibody levels, improving thyroid function, and improving other markers of autoimmunity, such as cytokines, e.g., IP10, TNF-α, and IL-10, and the ratio of T-cell subsets, such as Th17 and Tr1. Studies suggest that by impacting various immunological mechanisms, vitamin D may help control autoimmunity and improve thyroid function and, potentially, clinical outcomes of HT patients. The article discusses the potential impact of vitamin D on various immune pathways in HT. Overall, current evidence supports the potential role of vitamin D in the prevention and management of HT, although further studies are needed to fully understand its mechanisms of action and potential therapeutic benefits.
Keywords: Hashimoto’s thyroiditis, vitamin D, autoimmunity, cytokines, anti-thyroid antibodies
1. Introduction
Hashimoto’s thyroiditis (HT), also known as chronic lymphocytic thyroiditis and Hashimoto’s disease, is an autoimmune thyroid disease (AITD) with a complex etiopathology, which includes genetic (e.g., major histocompatibility complex (MHC) genes) and environmental factors, such as past infections, medications, and smoking, and the level of microelements, such as iodine, iron, and selenium [1,2]. With an incidence of 0.3–1.5 cases per 1000 people, HT is the most common cause of hypothyroidism in iodine-replete areas [3].
Despite much research on its immunopathology, HT remains an incurable disease with an unpredictable nature, often leading to lymphocytic destruction of the thyroid gland and the need for thyroid hormone replacement for life [4,5]. However, some studies showed that interventions within modifiable risk factors might improve immunological and clinical outcomes in HT patients. This includes, among other things, dietary changes, stress management, selenium supplementation, and vitamin D supplementation [6,7,8,9].
In this article, we aim to review the mechanisms of the immunomodulatory activity of vitamin D and its impact on the autoimmune process in Hashimoto’s thyroiditis.
2. Material and Methods
The systematic literature research used Pubmed, SCOPUS, and Web of Science databases. The following keywords were used, in combinations or individually: vitamin D, thyroiditis, Hashimoto, autoimmune thyroid disease, immune system, cytokines, and supplementation. The database search focused on original research and meta-analyses presenting studies conducted on humans and animals, published between January 1999 and March 2023. For the trials which would present the association between vitamin D concentration and immunological parameters in HT in humans, as well as changes in immune markers after vitamin D supplementation, we researched the last decade (2013–2023), excluding case reports, narrative reviews, editorials, and commentaries.
3. Vitamin D
3.1. Sources, Metabolism, and Function
Vitamin D is a fat-soluble vitamin that plays a crucial role in bone health and calcium regulation in the body. There are two main forms of vitamin D: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D2 is found in some plant foods, while vitamin D3 is synthesized in the skin upon exposure to sunlight [10]. When vitamin D is consumed or synthesized, it first undergoes hydroxylation in the liver, where it is converted into 25-hydroxyvitamin D (25(OH)D), which is commonly used as an indicator of vitamin D status in the body. Next, 25(OH)D is transported to the kidneys, where it undergoes a second hydroxylation step to become the biologically active form of vitamin D known as calcitriol, also known as 1,25-dihydroxyvitamin D (1,25(OH)2D) [11].
Calcitriol then binds to vitamin D receptors (VDRs) in various tissues throughout the body, including the bones, intestines, and kidneys, to regulate calcium and phosphate metabolism. In the intestines, calcitriol increases the absorption of calcium and phosphorus, while in the kidneys, it increases calcium reabsorption and phosphate excretion. Calcitriol helps regulate bone remodeling and mineralization, ensuring proper bone growth and maintenance [12].
In addition to its role in bone health, research has proved its pleiotropic effects which are significant for disease prevention [13]. Many studies have linked vitamin D to various health benefits, including mood regulation, reduced risk of chronic cardiovascular diseases, immune function, and others [14,15,16,17]. Proper levels of vitamin D are essential in the prevention of musculoskeletal [18], cardiovascular [19], dementia [20,21], cancer [22], autoimmune [15], metabolic [23], PCOS [22,24], or even kidney diseases [25]. Examples of pleiotropic actions with their clinical significance in different diseases are shown in Table 1.
Table 1.
Examples of possible pleiotropic actions of vitamin D and their potential clinical significance.
| Disease Group | Vitamin D Functions | Disease Prevention |
|---|---|---|
| Musculoskeletal diseases [18] |
|
|
| Cardiovascular diseases [19] |
|
|
| Brain diseases [20,21] |
|
|
| Cancers [22] |
|
|
| Immune-mediated diseases [15] |
|
|
| Metabolic diseases [23] |
|
|
| Female reproductive system diseases [22,24] |
|
PCOS |
| Renal system diseases [25] |
|
CKD |
AITD, autoimmune thyroid disease; BSP, bone sialoprotein; CKD, chronic kidney disease; DMP, dentin matrix acidic phosphoprotein; MS, multiple sclerosis; PCOS, polycystic ovaries syndrome; RA, rheumatoid arthritis; RAAS, renin–angiotensin–aldosterone system; SLE, systemic lupus erythematosus; VEGF, vascular endothelial growth factor.
The pleiotropic actions of vitamin D are mainly considered to come from acting on the genomic level, directly regulating gene expression via VDR/RXR (vitamin D receptor/retinoid X receptor) complex. However, the research shows it also acts via non-genomic, rapid pathways, altering gene expression through epigenetic mechanisms. For example, in the immune system, as described by Hii et al. [26], vitamin D can control VDR binding to target proteins like STAT1 (signal transducer and activator of transcription 1) and IKK-β (inhibitor of nuclear factor kappa-B kinase subunit beta). This function enables vitamin D to cross-modulate gene expression mediated by non-vitamin D ligands, such as TNF-α and IFN-γ. Vitamin D may exert control over immunological and antiviral responses through this mechanism. Other non-genomic mechanisms of vitamin D action, concerning, among other things, interaction with PDIA3 (protein disulfide isomerase family member 3) and rapid intracellular calcium regulation via L-VGCC (L-type voltage-gated calcium channel) are speculated to play a role in proper bone, muscle, and brain development [27,28].
While sunlight is the most efficient way to obtain vitamin D, it can also be found in certain foods such as fatty fish, egg yolks, dairy, and grain products [29]. However, obtaining enough vitamin D through diet or sunlight alone can be challenging for numerous high-risk groups, which include, among other things, individuals over 65 years of age, dark-skinned populations, patients with cancer, autoimmune diseases, malabsorption syndromes, cardiovascular diseases, diabetes, and people with body mass index (BMI) > 30 [30,31]. According to a recent pooled analysis of 7.9 million participants by Cui et al. [32], 15.7% of the global population is vitamin D deficient. At the same time, other studies indicate that the prevalence of vitamin D deficiency in Europe may be as high as 40%, and the prevalence of severe vitamin D deficiency—13% [33]. Because of the scale of the problem, the need for widespread correction of vitamin D status, mainly with supplementation, is often considered a public health problem [34].
3.2. The Role of Vitamin D in the Immune System
Vitamin D significantly impacts the immune system, as VDRs are present in many peripheral blood mononuclear cells, including T and B cells and antigen-presenting cells (APCs) [35]. Via genomic pathways, calcitriol influences the transcriptional activity of genes involved in immune cell functioning and regulates processes such as cell differentiation, the cell cycle, programmed cell death, stress response, and fighting infections. For example, Vitamin D may induce the expression of antibiotic peptides, such as CAMP/LL-37 (cathelicidin antimicrobial peptide LL-37), which destroy the cell membranes of bacteria and viruses [36]. Vitamin D also enhances autophagy in macrophages, which helps clear viruses from the cells, including SARS-CoV-2 [37].
Furthermore, vitamin D plays a significant role in preventing autoimmune processes [15,38,39]. Vitamin D downregulates MHC class II and co-stimulatory molecules expressed on dendritic cells (DCs), which are major APCs, thus preventing excessive T-cell activation [40]. Vitamin D also suppresses DC cytokine production and promotes the expression of anti-inflammatory cytokines, such as interleukin 1 (IL-10) [41]. In T cells, vitamin D suppresses the proliferation and differentiation of CD4+ T cells (helper T cells, Th cells) and promotes their differentiation into Th2 cells, which helps maintain Th1/Th2 balance [42]. Vitamin D also inhibits the development of Th17 cells and promotes the differentiation of regulatory T cells (Tregs) that prevent an increased autoimmune response by, among other things, secreting anti-inflammatory cytokines [43]. B cells, which produce antibodies, also express VDR. Vitamin D has been found to affect B cells in various ways, including the inhibition of naive B cell differentiation or maturation to plasma cells, which may potentially reduce autoantibody production [44].
It should be emphasized that the positive influence of vitamin D on the immune system at the cellular and molecular level also translates into improved clinical outcomes for patients with autoimmune diseases. Several clinical trials and observational studies have demonstrated that vitamin D supplementation may benefit the prevalence and disease activity of multiple autoimmune conditions, e.g., rheumatoid arthritis [45], inflammatory bowel disease [46], and vitiligo [47]. For example, the VITAL randomized controlled trial from 2022, which included 25,871 participants from the United States, showed that vitamin D supplementation of 2000 IU/day for five years reduced the risk of developing any autoimmune disease by 22% [48]. Multiple meta-analyses also proved that vitamin D deficiency increases the risk of developing various autoimmune conditions, including AITD [49,50,51,52,53].
4. Recent Findings in Hashimoto’s Thyroiditis Immunopathology
4.1. Etiological Factors Affecting the Development of HT
Despite many studies leading to a better understanding of autoimmune processes at the cellular and molecular level, the exact causes and triggers that initiate the thyroid destruction process in HT remain unknown. Multiple factors have been proven to impact the risk of HT, including exposure to environmental components (e.g., infections, irradiation, selenium), the presence of different diseases (e.g., diabetes, allergic rhinitis), and differentially expressed genes [54,55,56]. Genetic predisposition includes MHC genes (e.g., HLA-DR3) and genes regulating immune responses like IL-7R (Interleukin 7 receptor) gene, OR2J3 (olfactory receptor 2J3) gene, or CTLA4 (cytotoxic T-lymphocyte-associated protein 4) gene. This proves the complex etiology of the disease, for which there is still no effective causal treatment.
We know that the core of the autoimmune process in HT is a breakdown in self-tolerance to thyroid autoantigens, which leads to thyroid destruction by infiltration of CD4+ T cells, macrophages, and plasma cells, which produce auto-antibodies against thyroid peroxidase (anti-TPO) and thyroglobulin (anti-Tg) [57]. Therefore, the detection of elevated titers of these antibodies is generally used for HT diagnosis [58].
4.2. Immunopathological Processes in HT on the Level of Cells and Cytokines
From the morphopathological perspective, HT disease typically leads to thyroid enlargement with nodule development. In HT, there is an extensive infiltration of the parenchyma by a mononuclear inflammatory infiltrate containing small lymphocytes, plasma cells, and well-developed germinal centers. The CD4:CD8 ratio in the infiltrate is 4:1. The atrophy of the colloid bodies is lined by Hürthle cells [59,60].
Many studies show that, from the immunological viewpoint, the main contributors to the development of HT are (1) Th1/Th2 cell imbalance and (2) Th1 cell activity enhancement [16], which lead to disturbances in the complex interplay between different immune components. The characteristics of thyroid autoimmunity in HT on the cellular and molecular levels were reviewed by Luty et al. in detail [61].
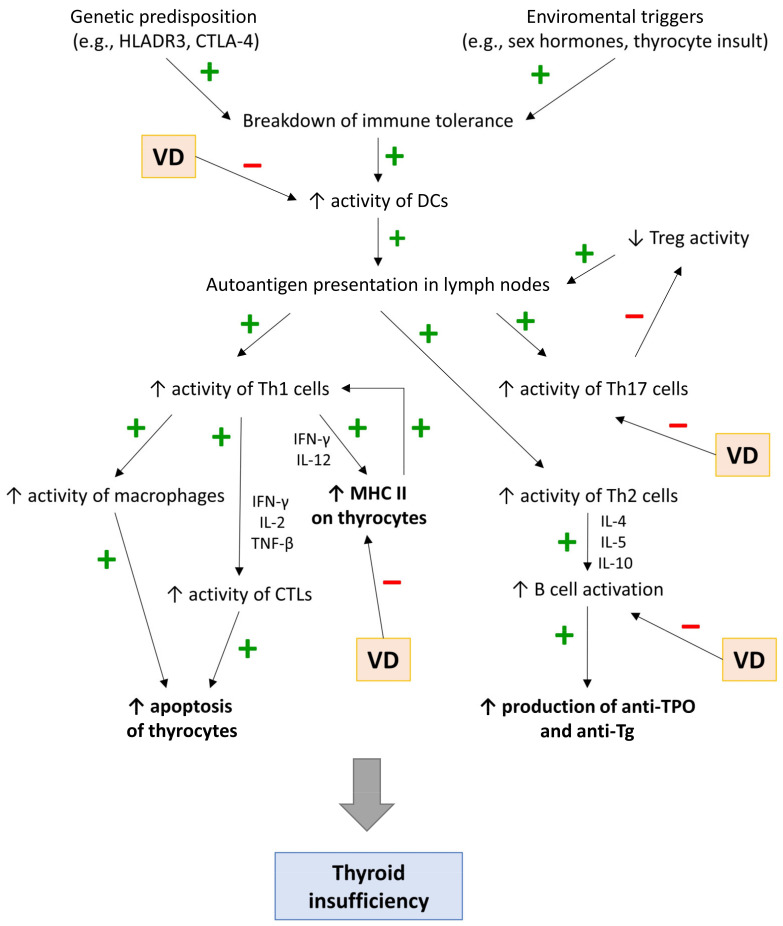
First, the presence of environmental/genetic factors leads to the activation of APCs, mainly DCs, which present allo- and autoantigens to naive CD4+ T cells, leading to their differentiation into Th1, Th2, Th17, Th22, or Tregs (Figure 1). Second, cytokines produced by Th1, including IL-12 and IFN-γ, induce the expression of MHC II on thyroid cells, further promoting the differentiation of the naive CD4+ T cells into Th1. Finally, Th1 cells, through IFN-γ, IL-2, and TGF-β, induce the activation of CD8+ T cells (cytotoxic T cells, Tc cells).
Figure 1.
Immunopathomechanisms of Hashimoto’s thyroiditis.
CD8+ T cells, through secreted perforins and granzymes or via Fas-FasL cascade [62], destroy thyroid cells, which leads to the release of proinflammatory cytokines and chemokines. This leads to an amplification feedback loop that initiates and sustains the immune process. Cytokines released by thyrocytes contribute to (1) the migration and activation of pathological Th17 cells (IL-6, TNF-α, IL-1β, and TGF-β) and (2) the suppression of Tregs (IL-6, IFN-γ, IL-8, IL-1β, and CXCL10), which usually inhibit excessive T-cell-mediated cytotoxicity [63]. Moreover, the microenvironment in the infiltrated thyroid promotes the differentiation of proinflammatory Th22 cells. The destruction of the thyroid gland and the strengthening of the autoimmune process also occur through the humoral response. Infiltrating B cells (triggered by Th2 cytokines, i.e., IL-4, IL-5, and IL-10) release autoantibodies (mainly anti-TPO and anti-Tg), which can further lead to the destruction of thyrocytes in the mechanism of antibody-dependent cell-mediated cytotoxicity (ADCC) [61].
5. Role of Vitamin D in the Immunopathology of Hashimoto’s Thyroiditis
5.1. Immunomodulatory Potential of Vitamin D in HT
Rui et al. [16] presented four potential mechanisms in which Vitamin D can contribute to the inhibition of the immune process in HT: (1) prevention of DC-dependent T-cell activation; (2) down-regulation of HLA class II gene expression in the thyroid; (3) influence on B-cells and (4) restoration of the Th17/Tregs ratio.
Most subsets of dendritic cells (e.g., conventional DCs and Langerhans cells) have vitamin D receptors on their surface [64]. It has been proven that the 1,25(OH)2D3-VDR complex can inhibit the expression of various proinflammatory cytokines from DCs that activate T cells (IL-2, IL-5, IL-17, IL-12, IL-23, IL-6, TNFα, INF-γ, CCL5, and CCL17) while enhancing the expression of IL-10 and IL-8. These effects may potentially contribute to the improvement of HT by (1) a shift from the Th1 profile towards Th2 (2), a decrease in pathological Th17 responses (3), and a decrease in a cytokine-mediated immune response [64,65].
In contrast to the healthy population, the follicular thyroid cells of HT patients may express MHC class II molecules, which are crucial for presenting antigens to CD4+ T cells. In that manner, thyroid cells can act as APCs by presenting autoantigens to T cells and activating them. Induction of MHC class II on follicular cells by, i.e., IFN-γ, and IL-12, can contribute to the autoimmune process in HT [61]. 1,25(OH)D can reduce MHC II expression, thus preventing T-cell activation and proinflammatory cytokine response [15].
B cells play a role in the pathogenesis of HT mainly by producing antibodies, anti-TPO, and anti-Tg, which are thyroid self-antigens. Antibodies contribute to the apoptosis of thyroid follicular cells in the mechanism of antibody-dependent cell-mediated cytotoxicity [66]. Although the particular role of vitamin D on ADCC in HT is not fully understood, it has been suggested that insufficient 25(OH)D concentration are associated with increased B-cell proliferation, differentiation, and antibody titers in autoimmune diseases, such as SLE and MS [67,68]. Vitamin D has been proven to exert inhibitory effects on plasma cell generation, which can, in turn, contribute to decreased immunoglobulin production [69]. Some studies demonstrated that 1,25D-treated Th cells may suppress B-cell differentiation, proliferation, and antibody production [40]. Many studies proved the association between low vitamin D status and antithyroid antibody titers and a decrease in antithyroid antibody titers after treating vitamin D deficiency. These effects have been confirmed in meta-analyses [53,70,71,72].
Although Th17 and Tregs differentiate from a common precursor, the naive CD4+ T cells, have different, even opposite, functions. Th17 cells express mainly proinflammatory activity, which contributes to the development of inflammation and autoimmune disorders, whereas Tregs modulate the immune system and maintain tolerance to self-antigens which prevents autoimmunity [73].
Many studies confirmed the critical role of increased Th17/Treg ratio in the pathogenesis of various autoimmune disorders, including AITD [74,75]. Vitamin D inhibits the differentiation of naive T cells into Th17 while increasing the levels of Tregs, which restores the Th17/Treg ratio in the body [76,77]. Moreover, vitamin D can inhibit the secretion of cytokines (mainly IL-17) from Th17 cells [78], potentially reducing the Th17 cell-induced thyroid inflammation.
5.2. Association between Vitamin D, the Occurrence of HT, and Antibody Levels
The association between vitamin D and HT remains controversial. Many studies to date investigated this topic in various populations, and the results still have some inconsistency. Some work, including observations from the Croatian Biobank of HT patients and other comparative studies, did not detect an association between vitamin D levels and the prevalence of HT [79,80,81]. However, large-scale studies, including systematic reviews and meta-analyses, confirm the association between low vitamin D and HT. Meta-analysis of observational studies by Taheriniya et al. [53] showed lower vitamin D levels in patients with HT than in healthy subjects. Similar results were shown in meta-analyses by Wang et al. [71] and Štefanić and Tokić [72]; HT patients were more likely to have lower 25(OH)D than a healthy population. In studies by Kim et al. [82,83] on the Korean population, including a nationwide survey of 4181 participants, vitamin D deficiency was significantly associated with a high prevalence of thyroid autoimmunity. Other studies showed that low 25(OH)D correlates with HT in children and adolescents [84], as well as in obese subjects [85]. Moreover, some studies suggest an association between serum 25(OH)D levels and the clinical presentation of HT, including the severity of hypothyroidism and the prevalence of mild cognitive impairment [79,86].
Apart from the correlation between vitamin D status and the prevalence of HT, several studies investigated the association between vitamin D and antithyroid antibody levels. In the comparative study by Aktaş et al. [87], there was a negative correlation between 25(OH)D level and anti-TPO in 130 patients diagnosed with HT. Similar results were obtained by Bozkurt et al. [88], who also demonstrated that vitamin D deficiency severity correlated with the duration of HT, thyroid volume, and antibody levels. Interestingly, Sayki Arslan et al. [89] also discovered that anti-TPO positivity was significantly more common in healthy subjects (HT not diagnosed) with vitamin D deficiency compared to those with a normal 25(OH)D level.
5.3. Association between Vitamin D Levels and Immunological Parameters in HT
Most studies that evaluated the immunity of HT concerning vitamin D focus on thyroid autoantibody titers as markers of the autoimmunological process. However, the potential impact of vitamin D may concern various other immunological mechanisms. Few studies assessed the association of vitamin D and HT in the context of different immune pathways, with varying results. For example, in HT patients, Botehlo et al. [90] showed no significant correlation between 25(OH)D status and IL-2, IL-4, and IFN-γ serum levels. However, a positive correlation was observed between vitamin D and IL-17, TNF-α, and IL-5. According to the authors, the lower TNF-α and IL-17 levels, which correlated with low vitamin D status, could be explained by the control of cytotoxicity by long-time treatment of HT. As stated by Korzeniowska et al. [91], levothyroxine may contribute to stabilizing the inflammatory process in HT. In a study by Wencai Ke et al. [92], serum 25(OH)D levels were not associated with IL-4, IL-17, and TNF-α in newly diagnosed or treated patients with HT. However, vitamin D concentrations were relatively deficient in those subjects.
Feng et al. [93] discovered that, amongst Chinese children with HT, serum IL-21 concentration was positively correlated with antithyroid antibodies, while the serum concentration of 25(OH)D had a significant negative correlation with serum IL-21. According to the investigators, the results showed that vitamin D levels and IL-21 might be involved in the occurrence and development of HT.
It is worth noting that the results by Hisbiyah et al. [94], who investigated children with Down syndrome and HT. Contrary to most studies, they found a positive correlation between vitamin D and antithyroid antibody levels. They also found no correlation between vitamin D and NF-κB (nuclear factor-kappa B), suggesting that vitamin D could not affect NF-κB, a transcription factor whose pathway, according to Giuliani et al. [95], can also play a role in thyroid autoimmunity. However, the authors concluded that vitamin D could suppress IFN-γ, which is involved in, i.e., the suppression of Tregs expression and the activation of CD8+ T cells in HT. [61]
Roehlen et al. [96] presented even more insights into the mechanisms of vitamin D immunoregulation. They investigated if the immunoregulatory function of vitamin D can be related to FOXO3a gene polymorphisms and SIRT1 (sirtuin 1 histone deacetylase) in HT and DTC (differentiated thyroid cancer). SIRT1 and FOXO3a are proteins that regulate cellular processes related to aging and disease [97]. FOXO3a is a transcription factor that regulates the expression of multiple genes associated with, i.e., cell proliferation, apoptosis, and cellular stress [98], while SIRT1 is a deacetylase that modifies the activity of various proteins, e.g., p53, NF-κB, and FOXO3a [99]. SIRT1 activates FOXO3a through deacetylation, leading to the upregulation of genes involved in stress resistance and longevity. In vitro, vitamin D exerted an anti-proliferative effect in Th cells that was blocked by SIRT1 inhibition and accompanied by elevated FOXO3a gene expression. The authors concluded that the SIRT1-FOXO3a axis is one of the downstream targets of vitamin D immunoregulatory effects. Moreover, they identified two single nucleotide polymorphisms in the FOXO3a gene (rs9400239T and rs4945816C) that may constitute genetic risk factors for HT [96].
Tokic et al. [100] discovered that T cells from HT patients exhibited lower CTLA4, CD28, and CD45RAB gene expression than healthy controls. All these molecules may play a role in thyroid autoimmunity. The CD28/T-cell receptor (TCR)/CTLA4 complex regulates T-cell homeostasis and tolerance in HT, while CD45 protein tyrosine phosphatase (PTPase) cooperates with the vitamin D receptor to interact with the TCR complex and influence the Th1/Th17/Treg pathways that are critical to the development of HT.
The association between vitamin D status, antithyroid antibody levels, and different immunological parameters in different studies is summarized in Table 2.
Table 2.
The association between vitamin D status, antithyroid antibody levels, and different immunological parameters in different studies.
| Author, Year | Type of Study | Number of Participants | Main Findings |
|---|---|---|---|
| Bozkurt et al., 2013 [88] | Case–control study |
|
|
| Evliyaoğlu et al., 2015 [84] | Case–control study |
|
The prevalence of 25(OH)D deficiency in HT patients was significantly higher than that in the control group |
| Arslan et al., 2015 [89] | Cross-sectional study | 155 HC |
|
| Wang et al., 2015 [71] | Meta-analysis | (a) The continuous 25(OH)D by AITD * status:
|
HT patients had lower 25(OH)D levels and were more likely to have a 25(OH)D deficiency |
| Kim et al., 2016 [82] | Retrospective cross-sectional study | 776 HT patients with measured 25(OH)D | 25(OH)D insufficiency was associated with HT, especially overt hypothyroidism |
| Kim et al., 2017 [83] | Cross-sectional study; a nationwide survey | 4181 participants | Anti-TPO positivity was more prevalent in the 25(OH)D deficient group than in insufficient and sufficient 25(OH)D groups |
| Wencai Ke et al., 2017 [92] | Cross-sectional study |
|
25(OH)D levels were not associated with thyroid function, antithyroid antibodies, and serum cytokines IL-4, IL-17, and TNF-α in patients with AITD * |
| Tokic et al., 2017 [100] | Cross-sectional study |
|
Nominally higher expression levels of VDR mRNA were found in T cells of healthy controls when compared to the HT patients |
| De Pergola et al., 2018 [85] | Cross-sectional study | 261 overweight and obese subjects | 25(OH)D deficiency is significantly associated with HT in overweight and obese subjects |
| Botelho et al., 2018 [90] | Cross-sectional study |
|
A positive correlation between 25(OH)D and fT4, IL-17, TNF-α and IL-5 in HT group |
| Jun Xu et al., 2018 [86] | Case–control study |
|
|
| Aktaş, 2019 [87] | Retrospective cohort study | 130 HT patients |
|
| Feng et al., 2020 [93] | Cross-sectional study |
|
|
| Štefanić and Tokić, 2020 [55] | Meta-analysis |
|
Lower serum 25(OH) in HT compared to healthy controls |
| Cvek et al., 2021 [79] | Case–control study; observations from biobank data |
|
|
| Hanna et al., 2021 [80] | Case–control study |
|
25(OH)D level was statistically indifferent between HT and control groups |
| Taheriniya et al., 2021 [53] | Meta-analysis |
|
Significantly lower 25(OH)D level among HT patients compared to healthy controls |
| Hisbiyah et al., 2022 [94] | Cross-sectional study | 80 Down syndrome patients (children) |
|
| Filipova et al., 2023 [81] | Prospective case–control study |
|
No significant association between 25(OH)D and thyroid autoantibodies, thyroid hormones, and thyroid volume |
25(OH)D, 25-hydroxycholecalciferol; AITD, autoimmune thyroid disease; anti-TPO, antithyroid peroxidase antibodies; fT4, free thyroxine; GD, Graves’ disease; HC, healthy controls; HT, Hashimoto’s thyroiditis; MCI, mild cognitive impairment; TRAb, TSH receptor antibody; VD, vitamin D; * AITD patients included Graves’ disease and Hashimoto’s thyroiditis patients.
5.4. Changes in Immunological Parameters and HT Outcomes after Vitamin D Supplementation
Several studies investigated immunological and inflammatory changes after vitamin D supplementation in patients with HT. Most results show a significant improvement in markers of immunity after the intervention. Krysiak et al. [101,102,103] conducted experiments amongst various HT populations in Poland (e.g., men with HT and alopecia [101], men with HT and testosterone deficiency [102], and euthyroid women with HT [102,103]), in which they assessed anti-TPO and anti-Tg levels after six months of 2000–4000 IU daily vitamin D supplementation. In these studies, 25(OH)D concentration increased (often achieving levels exceeding 30 ng/mL, which are adequate, according to Polish, as well as Central and European guidelines [104,105], and antithyroid antibody levels significantly decreased. The reduction in antibody titers was also present in the subjects with normal baseline vitamin D status [7]. In addition, researchers discovered many factors that impacted the effect of vitamin D on antithyroid antibodies. Dehydroepiandrosterone, simvastatin, and selenomethionine potentiated the effect, while hyperprolactinemia and a gluten-free diet (GFD) attenuated it [103,106,107,108,109]. GFD inhibiting the reduction in antithyroid antibodies by vitamin D is particularly interesting, as it adds another argument to the discussion about whether a gluten-free diet should be considered for non-celiac autoimmune disorders, which remains a controversial topic [110,111]. Authors explained that GFD patients might have ingested smaller amounts of unsaturated fatty acids, iron, and calcium, contributing to proper intestinal vitamin D absorption [109].
Other studies in different populations also obtained a reduction in antithyroid antibody levels after vitamin D supplementation. For example, Mazokopakis et al. [112] achieved a significant reduction in anti-TPO and anti-Tg with doses from 1200 to 4000 IU every day for 4 months (aiming to maintain serum 25(OH)D levels of 40 ng/mL) in Greek patients. Similar results were also obtained in Indian patients (60,000 IU weekly for 8 weeks reduced anti-TPO by 46.73%) [113] and amongst Iranian children with HT [114]. Noteworthy are also the results from Sinsek et al. [115], who demonstrated a significant reduction in anti-TPO and anti-Tg amongst Turkish HT and GD patients with only one month of 1000 IU daily vitamin D. A meta-analysis by Zhang et al. [70] also showed that vitamin D supplementation reduces anti-TPO and anti-Tg titers, especially with a supplementation duration of over 3 months.
Particularly intriguing are the outcomes of the community-based program conducted in Canada. The program database included 11,017 participants that were provided with vitamin D to achieve 25(OH)D concentrations exceeding 100 nmol/L (>40 ng/mL). After 12 months of follow-up, a significant reduction in antithyroid antibodies was observed [116]. Among subjects with elevated antithyroid antibody levels at baseline and follow-up, 77.5% were within the reference range for anti-TG and 42.2% for anti-TPO. In addition, serum 25(OH)D concentrations ≥ 125 nmol/L were associated with a 32% reduced risk of elevated antithyroid antibodies. Moreover, achieving correct vitamin D concentration was associated with substantial improvement in thyroid function, including reduced TSH levels and the severity of symptoms. This effect was especially amongst subjects with subclinical hypothyroidism, which was reduced by 72% at follow-up.
Although most studies on antithyroid antibodies after vitamin D gave consistent outcomes, it should be mentioned that some investigations showed contrary results. Vondra et al. [117] detected no decrease in antithyroid antibodies after three months of 4300 IU/day 25(OH)D supplementation, which could be explained by their relatively low initial levels. However, in a study by Behera et al. [118], amongst 23 HT patients from a coastal province of India, a significant increase in anti-TPO occurred after weekly 60,000 IU vitamin D supplementation for 8 weeks. The authors stated that they were unable to explain this effect.
Besides antibody levels, several researchers explored how vitamin D supplementation can affect other immunological markers. Robat-Jazi et al. [119] supplemented 40 HT female patients with 50,000 IU of 25(OH)D weekly for 3 months. At the baseline and follow-up, they assessed the serum levels of inflammatory factors, such as TNF-α, IFN-γ, and the chemokine CXCL10 (or IP10, interferon gamma-induced protein 10). IP10 is involved in the activation of T cells and the regulation of immune cell migration and proliferation. It may also contribute to the breakdown of immune tolerance by promoting the activation of autoreactive T cells in the thyroid [61,120]. IP10 is elevated in several other autoimmune diseases, including RA, MS, and SLE [121]. In this study, the serum levels of IFN-γ, TNF-α, and IP10 decreased significantly after the intervention suggesting the potential of vitamin D to control the inflammatory axis of IFNγ-IP10 and TNF-α in HT. A clinical trial conducted by Nodehi et al. [122] assessed the frequency of CD4+ T-cell subsets before and after the 3-month supplementation of 50,000 IU weekly vitamin D. The supplementation provided beneficial immunological effects by causing a significant decrease in Th17/Tr1 (regulatory T cells type 1) ratio. However, the activity of IL-10 in Tr1 cells increased in the vitamin D group, compared to that in the placebo group, with no statistical significance.
The changes in antithyroid antibody levels and different immunological parameters after vitamin D supplementation in different studies are summarized in Table 3.
Table 3.
Changes in antithyroid antibody levels and different immunological parameters after vitamin D supplementation in different studies.
| Author, Year | Dose and Supplementation Duration | Changes in 25(OH)D Levels | Other Changes in Immunological Parameters | Changes in Anti-TPO Titers | Changes in Anti-Tg Titers |
|---|---|---|---|---|---|
| Mazokopakis et al., 2015 [112] | 1200–4000 IU daily, aiming to achieve 25(OH)D concentration of >40 ng/mL, 4 months | ⬆ | ⬇ | ⬇ | |
| Chaudhary et al., 2016 [113] | 60,000 IU weekly, 8 weeks | ⬆ | ⬇ | ||
| Simsek et al., 2016 [115] | 1000 IU daily, 1 month | ⬆ | ⬇ | ⬇ | |
| Mirhosseini et al., 2017 [116] | Doses modified with the aim to achieve 25(OH)D concentration of >40 ng/mL, 1 year | ⬆ | CRP ⬇ | ⬇ | ⬇ |
| Vondra et al., 2017 [117] | 4300 IU daily, 3 months | ⬆ | CRP ⬌ | ⬆ | ⬆ |
| Nodehi et al., 2019 [122] | 50,000 IU weekly, 3 months | ⬆ |
|
||
| Aghili et al., 2020 [114] | Varied depending on initial and rechecked 25(OH)D concentrations | ⬆ | ⬇ | ⬇ | |
| Behera et al., 2020 [118] | 60,000 IU weekly, 2 months, then 60,000 IU monthly, 4 months | ⬆ | ⬆ | ||
| Krysiak et al., 2016–2022 [7,101,102,103,106,107,108] | 2000–4000 IU daily for 6 months | ⬆ | ⬇ | ⬇ | |
| Krysiak et al., 2022 [109] | 4000 IU daily for 6 months | ⬆ | CRP ⬌ | ⬇ | ⬇ |
| Robat-Jazi et al., 2022 [119] | 50,000 IU weekly, 3 months | ⬆ |
|
⬇ |
25(OH)D, 25-hydroxycholecalciferol; CRP, C-reactive protein; IL-10; interleukin 10; IFN-γ, interferon gamma; IP10, interferon gamma-induced protein 10; Th17, T helper 17 cell; TNF-α, tumor necrosis factor alpha; Tr1, type 1 regulatory T cell; ⬆, an increase; ⬇, a decrease; ⬌, no significant change.
6. Conclusions
In conclusion, the relationship between vitamin D and Hashimoto’s thyroiditis has been the subject of numerous studies with varying results. Recent findings indicate that vitamin D may have an immunomodulatory effect on HT by, among other things, influencing DC-dependent T-cell activation, downregulating HLA class II gene expression in the thyroid, preventing excessive B-cell response, and balancing the Th17/Treg cell ratio. While laboratory insights into vitamin D function in HT are valuable, it is also crucial to examine the real-world clinical outcomes, including associations between vitamin D and symptoms, thyroid, and immune system function, as well as the impact of vitamin D on disease manifestation and progression. While some research has shown no association between 25(OH)D concentration and the prevalence of HT, several large-scale studies, systematic reviews, and meta-analyses have confirmed a link between low 25(OH)D levels and HT. Additionally, numerous studies have demonstrated a negative correlation between vitamin D levels and antithyroid antibody levels.
Besides the correlation between vitamin D status and HT prevalence, research has explored the association between vitamin D and other immunological parameters in HT, such as cytokines and T-cell subsets. While the results have been mixed, some studies have shown a significant negative correlation between 25(OH)D concentration and inflammatory cytokines.
Research on the effects of vitamin D supplementation in HT patients has shown promising results, with most studies reporting a significant improvement in immunological markers after the intervention. However, a few studies have yielded contrasting findings. Overall, the available evidence suggests that vitamin D supplementation may have beneficial immunomodulatory effects in HT patients, but further research is necessary to determine the optimal dosing, duration, and potential interactions with other treatments or dietary factors.
Future studies need to continue investigating the complex relationship between vitamin D and HT and the impact of vitamin D supplementation on various immunological markers and clinical outcomes. This knowledge will contribute to a better understanding of the role of vitamin D in HT pathogenesis and inform potential therapeutic strategies for individuals with HT.
Author Contributions
F.L.: conceptualization, methodology, writing original draft, K.A.L.: review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the statutory funds of the Medical University of Gdańsk (grant number 02-0058/07).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Weetman A.P. An Update on the Pathogenesis of Hashimoto’s Thyroiditis. J. Endocrinol. Investig. 2021;44:883–890. doi: 10.1007/s40618-020-01477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szklarz M., Gontarz-Nowak K., Matuszewski W., Bandurska-Stankiewicz E. Iron: Not Just a Passive Bystander in AITD. Nutrients. 2022;14:4682. doi: 10.3390/nu14214682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralli M., Angeletti D., Fiore M., D’Aguanno V., Lambiase A., Artico M., de Vincentiis M., Greco A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020;19:102649. doi: 10.1016/j.autrev.2020.102649. [DOI] [PubMed] [Google Scholar]
- 4.Ihnatowicz P., Drywień M., Wątor P., Wojsiat J. The Importance of Nutritional Factors and Dietary Management of Hashimoto’s Thyroiditis. Ann. Agric. Environ. Med. 2020;27:184–193. doi: 10.26444/aaem/112331. [DOI] [PubMed] [Google Scholar]
- 5.Ransing R.S., Mishra K.K., Sarkar D. Neuropsychiatric Manifestation of Hashimoto’s Encephalopathy in an Adolescent and Treatment. Indian J. Psychol. Med. 2016;38:357–360. doi: 10.4103/0253-7176.185950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markomanolaki Z.S., Tigani X., Siamatras T., Bacopoulou F., Tsartsalis A., Artemiadis A., Megalooikonomou V., Vlachakis D., Chrousos G.P., Darviri C. Stress Management in Women with Hashimoto’s Thyroiditis: A Randomized Controlled Trial. J. Mol. Biochem. 2019;8:3–12. [PMC free article] [PubMed] [Google Scholar]
- 7.Krysiak R., Szkróbka W., Okopień B. The Effect of Vitamin D on Thyroid Autoimmunity in Levothyroxine-Treated Women with Hashimoto’s Thyroiditis and Normal Vitamin D Status. Exp. Clin. Endocrinol. Diabetes. 2017;125:229–233. doi: 10.1055/s-0042-123038. [DOI] [PubMed] [Google Scholar]
- 8.Krysiak R., Szkróbka W., Okopień B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes. 2019;127:417–422. doi: 10.1055/a-0653-7108. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y., Feng W., Chen H., Shi H., Jiang L., Zheng X., Liu X., Zhang W., Ge Y., Liu Y., et al. Effect of Selenium on Thyroid Autoimmunity and Regulatory T Cells in Patients with Hashimoto’s Thyroiditis: A Prospective Randomized-Controlled Trial. Clin. Transl. Sci. 2021;14:1390–1402. doi: 10.1111/cts.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janoušek J., Pilařová V., Macáková K., Nomura A., Veiga-Matos J., Silva D.D.d., Remião F., Saso L., Malá-Ládová K., Malý J., et al. Vitamin D: Sources, Physiological Role, Biokinetics, Deficiency, Therapeutic Use, Toxicity, and Overview of Analytical Methods for Detection of Vitamin D and Its Metabolites. Crit. Rev. Clin. Lab. Sci. 2022;59:517–554. doi: 10.1080/10408363.2022.2070595. [DOI] [PubMed] [Google Scholar]
- 11.Wierzbicka A., Oczkowicz M. Sex Differences in Vitamin D Metabolism, Serum Levels and Action. Br. J. Nutr. 2022;128:2115–2130. doi: 10.1017/S0007114522000149. [DOI] [PubMed] [Google Scholar]
- 12.Matikainen N., Pekkarinen T., Ryhänen E.M., Schalin-Jäntti C. Physiology of Calcium Homeostasis: An Overview. Endocrinol. Metab. Clin. N. Am. 2021;50:575–590. doi: 10.1016/j.ecl.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Egierska D., Pietruszka P., Burzyńska P., Chruścicka I., Buchta J. Pleiotropic Effects of Vitamin D3. J. Educ. Health Sport. 2021;11:143–155. doi: 10.12775/JEHS.2021.11.07.013. [DOI] [Google Scholar]
- 14.Huiberts L.M., Smolders K.C.H.J. Effects of Vitamin D on Mood and Sleep in the Healthy Population: Interpretations from the Serotonergic Pathway. Sleep Med. Rev. 2021;55:101379. doi: 10.1016/j.smrv.2020.101379. [DOI] [PubMed] [Google Scholar]
- 15.Lisowska K.A., Bryl E. The role of vitamin D in the development of autoimmune diseases. Postep. Hig. Med. Dosw. 2017;71:797–810. doi: 10.5604/01.3001.0010.3857. [DOI] [PubMed] [Google Scholar]
- 16.Zhao R., Zhang W., Ma C., Zhao Y., Xiong R., Wang H., Chen W., Zheng S.G. Immunomodulatory Function of Vitamin D and Its Role in Autoimmune Thyroid Disease. Front. Immunol. 2021;12:574967. doi: 10.3389/fimmu.2021.574967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latic N., Erben R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020;21:6483. doi: 10.3390/ijms21186483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendes M.M., Botelho P.B., Ribeiro H. Vitamin D and Musculoskeletal Health: Outstanding Aspects to Be Considered in the Light of Current Evidence. Endocr. Connect. 2022;11:e210596. doi: 10.1530/EC-21-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin K., Appel L.J., Michos E.D. Vitamin D, Calcium, and Cardiovascular Disease: A “D” Vantageous or “D” Etrimental? An Era of Uncertainty. Curr. Atheroscler. Rep. 2017;19:5. doi: 10.1007/s11883-017-0637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui X., McGrath J.J., Burne T.H.J., Eyles D.W. Vitamin D and Schizophrenia: 20 Years on. Mol. Psychiatry. 2021;26:2708–2720. doi: 10.1038/s41380-021-01025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Littlejohns T.J., Henley W.E., Lang I.A., Annweiler C., Beauchet O., Chaves P.H.M., Fried L., Kestenbaum B.R., Kuller L.H., Langa K.M., et al. Vitamin D and the Risk of Dementia and Alzheimer Disease. Neurology. 2014;83:920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szeja N. Pleiotropic effects of vitamin D—A review of current studies. Pomeranian J. Life Sci. 2017;63:128–134. doi: 10.21164/pomjlifesci.347. [DOI] [Google Scholar]
- 23.Cândido F.G., Bressan J. Vitamin D: Link between Osteoporosis, Obesity, and Diabetes? Int. J. Mol. Sci. 2014;15:6569–6591. doi: 10.3390/ijms15046569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skowrońska P., Pastuszek E., Kuczyński W., Jaszczoł M., Kuć P., Jakiel G., Wocławek-Potocka I., Łukaszuk K. The Role of Vitamin D in Reproductive Dysfunction in Women—A Systematic Review. Ann. Agric. Environ. Med. 2016;23:671–676. doi: 10.5604/12321966.1226865. [DOI] [PubMed] [Google Scholar]
- 25.Kim C.S., Kim S.W. Vitamin D and Chronic Kidney Disease. Korean J. Intern. Med. 2014;29:416–427. doi: 10.3904/kjim.2014.29.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hii C.S., Ferrante A. The Non-Genomic Actions of Vitamin D. Nutrients. 2016;8:135. doi: 10.3390/nu8030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Żmijewski M.A. Nongenomic Activities of Vitamin D. Nutrients. 2022;14:5104. doi: 10.3390/nu14235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui X., Gooch H., Petty A., McGrath J.J., Eyles D. Vitamin D and the Brain: Genomic and Non-Genomic Actions. Mol. Cell. Endocrinol. 2017;453:131–143. doi: 10.1016/j.mce.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Borel P., Caillaud D., Cano N.J. Vitamin D Bioavailability: State of the Art. Crit. Rev. Food Sci. Nutr. 2015;55:1193–1205. doi: 10.1080/10408398.2012.688897. [DOI] [PubMed] [Google Scholar]
- 30.Parva N.R., Tadepalli S., Singh P., Qian A., Joshi R., Kandala H., Nookala V.K., Cheriyath P. Prevalence of Vitamin D Deficiency and Associated Risk Factors in the US Population (2011–2012) Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bleizgys A. Vitamin D Dosing: Basic Principles and a Brief Algorithm (2021 Update) Nutrients. 2021;13:4415. doi: 10.3390/nu13124415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui A., Zhang T., Xiao P., Fan Z., Wang H., Zhuang Y. Global and Regional Prevalence of Vitamin D Deficiency in Population-Based Studies from 2000 to 2022: A Pooled Analysis of 7.9 Million Participants. Front. Nutr. 2023;10:1070808. doi: 10.3389/fnut.2023.1070808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amrein K., Scherkl M., Hoffmann M., Neuwersch-Sommeregger S., Köstenberger M., Tmava Berisha A., Martucci G., Pilz S., Malle O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020;74:1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palacios C., Gonzalez L. Is Vitamin D Deficiency a Major Global Public Health Problem? J. Steroid Biochem. Mol. Biol. 2014;144:138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bizzaro G., Antico A., Fortunato A., Bizzaro N. Vitamin D and Autoimmune Diseases: Is Vitamin D Receptor (VDR) Polymorphism the Culprit? Isr. Med. Assoc. J. 2017;19:438–443. [PubMed] [Google Scholar]
- 36.Ismailova A., White J.H. Vitamin D, Infections and Immunity. Rev. Endocr. Metab. Disord. 2022;23:265–277. doi: 10.1007/s11154-021-09679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilani S.J., Bin-Jumah M.N., Nadeem M.S., Kazmi I. Vitamin D Attenuates COVID-19 Complications via Modulation of Proinflammatory Cytokines, Antiviral Proteins, and Autophagy. Expert Rev. Anti-Infect. Ther. 2022;20:231–241. doi: 10.1080/14787210.2021.1941871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison S.R., Li D., Jeffery L.E., Raza K., Hewison M. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif. Tissue Int. 2020;106:58–75. doi: 10.1007/s00223-019-00577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ao T., Kikuta J., Ishii M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules. 2021;11:1624. doi: 10.3390/biom11111624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bscheider M., Butcher E.C. Vitamin D Immunoregulation through Dendritic Cells. Immunology. 2016;148:227–236. doi: 10.1111/imm.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao H., Wei X., Yang X. A Novel Update on Vitamin D in Recurrent Pregnancy Loss. Mol. Med. Rep. 2021;23:382. doi: 10.3892/mmr.2021.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cyprian F., Lefkou E., Varoudi K., Girardi G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front. Immunol. 2019;10:2739. doi: 10.3389/fimmu.2019.02739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rolf L., Muris A.-H., Hupperts R., Damoiseaux J. Illuminating Vitamin D Effects on B Cells—The Multiple Sclerosis Perspective. Immunology. 2016;147:275–284. doi: 10.1111/imm.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandrashekara S., Patted A. Role of Vitamin D Supplementation in Improving Disease Activity in Rheumatoid Arthritis: An Exploratory Study. Int. J. Rheum. Dis. 2017;20:825–831. doi: 10.1111/1756-185X.12770. [DOI] [PubMed] [Google Scholar]
- 46.El Amrousy D., El Ashry H., Hodeib H., Hassan S. Vitamin D in Children with Inflammatory Bowel Disease: A Randomized Controlled Clinical Trial. J. Clin. Gastroenterol. 2021;55:815–820. doi: 10.1097/MCG.0000000000001443. [DOI] [PubMed] [Google Scholar]
- 47.Karagüzel G., Sakarya N.P., Bahadır S., Yaman S., Ökten A. Vitamin D Status and the Effects of Oral Vitamin D Treatment in Children with Vitiligo: A Prospective Study. Clin. Nutr. ESPEN. 2016;15:28–31. doi: 10.1016/j.clnesp.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Hahn J., Cook N.R., Alexander E.K., Friedman S., Walter J., Bubes V., Kotler G., Lee I.-M., Manson J.E., Costenbader K.H. Vitamin D and Marine Omega 3 Fatty Acid Supplementation and Incident Autoimmune Disease: VITAL Randomized Controlled Trial. BMJ. 2022;376:e066452. doi: 10.1136/bmj-2021-066452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang M., Wu H., Zhao M., Long H., Lu Q. Vitamin D Status in Patients with Autoimmune Bullous Dermatoses: A Meta-Analysis. J. Dermatol. Treat. 2022;33:1356–1367. doi: 10.1080/09546634.2020.1810606. [DOI] [PubMed] [Google Scholar]
- 50.Lee S., Kim B.J., Lee C.H., Lee W.S. Increased Prevalence of Vitamin D Deficiency in Patients with Alopecia Areata: A Systematic Review and Meta-Analysis. J. Eur. Acad. Dermatol. Venereol. 2018;32:1214–1221. doi: 10.1111/jdv.14987. [DOI] [PubMed] [Google Scholar]
- 51.Upala S., Sanguankeo A. Low 25-Hydroxyvitamin D Levels Are Associated with Vitiligo: A Systematic Review and Meta-Analysis. Photodermatol. Photoimmunol. Photomed. 2016;32:181–190. doi: 10.1111/phpp.12241. [DOI] [PubMed] [Google Scholar]
- 52.Guan S.-Y., Cai H.-Y., Wang P., Lv T.-T., Liu L.-N., Mao Y.-M., Zhao C.-N., Wu Q., Dan Y.-L., Sam N.B., et al. Association between Circulating 25-Hydroxyvitamin D and Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Int. J. Rheum. Dis. 2019;22:1803–1813. doi: 10.1111/1756-185X.13676. [DOI] [PubMed] [Google Scholar]
- 53.Taheriniya S., Arab A., Hadi A., Fadel A., Askari G. Vitamin D and Thyroid Disorders: A Systematic Review and Meta-Analysis of Observational Studies. BMC Endocr. Disord. 2021;21:171. doi: 10.1186/s12902-021-00831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rayman M.P. Multiple Nutritional Factors and Thyroid Disease, with Particular Reference to Autoimmune Thyroid Disease. Proc. Nutr. Soc. 2019;78:34–44. doi: 10.1017/S0029665118001192. [DOI] [PubMed] [Google Scholar]
- 55.Li P., Liu F., Zhao M., Xu S., Li P., Cao J., Tian D., Tan Y., Zheng L., Cao X., et al. Prediction Models Constructed for Hashimoto’s Thyroiditis Risk Based on Clinical and Laboratory Factors. Front. Endocrinol. 2022;13:886953. doi: 10.3389/fendo.2022.886953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu K., Li K., Zeng T., Liao Y., Min J., Zhang N., Peng M., Kong W., Chen L. Integrative Analyses of Genes Associated with Hashimoto’s Thyroiditis. J. Immunol. Res. 2021;2021:8263829. doi: 10.1155/2021/8263829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Q.-Y., Ye X.-P., Zhou Z., Zhu C.-F., Li R., Fang Y., Zhang R.-J., Li L., Liu W., Wang Z., et al. Lymphocyte Infiltration and Thyrocyte Destruction Are Driven by Stromal and Immune Cell Components in Hashimoto’s Thyroiditis. Nat. Commun. 2022;13:775. doi: 10.1038/s41467-022-28120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ragusa F., Fallahi P., Elia G., Gonnella D., Paparo S.R., Giusti C., Churilov L.P., Ferrari S.M., Antonelli A. Hashimotos’ Thyroiditis: Epidemiology, Pathogenesis, Clinic and Therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019;33:101367. doi: 10.1016/j.beem.2019.101367. [DOI] [PubMed] [Google Scholar]
- 59.Akamizu T., Amino N. Hashimoto’s Thyroiditis. In: Feingold K.R., Anawalt B., Blackman M.R., Boyce A., Chrousos G., Corpas E., de Herder W.W., Dhatariya K., Hofland J., Dungan K., et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA, USA: 2000. [Google Scholar]
- 60.Chandanwale S.S., Kulkarni T.V., Patel R.J., Thakkar D. A Focal Nodular Hürthle Cell Hyperplasia in Hashimoto’s Thyroiditis: A Diagnostic Dilemma on Fine Needle Aspiration. J. Cytol. 2014;31:236–238. doi: 10.4103/0970-9371.151145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luty J., Ruckemann-Dziurdzińska K., Witkowski J.M., Bryl E. Immunological Aspects of Autoimmune Thyroid Disease—Complex Interplay between Cells and Cytokines. Cytokine. 2019;116:128–133. doi: 10.1016/j.cyto.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Stassi G., Todaro M., Bucchieri F., Stoppacciaro A., Farina F., Zummo G., Testi R., De Maria R. Fas/Fas Ligand-Driven T Cell Apoptosis as a Consequence of Ineffective Thyroid Immunoprivilege in Hashimoto’s Thyroiditis. J. Immunol. 1999;162:263–267. doi: 10.4049/jimmunol.162.1.263. [DOI] [PubMed] [Google Scholar]
- 63.Rocamora-Reverte L., Melzer F.L., Würzner R., Weinberger B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2021;11:616949. doi: 10.3389/fimmu.2020.616949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barragan M., Good M., Kolls J.K. Regulation of Dendritic Cell Function by Vitamin D. Nutrients. 2015;7:8127–8151. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Konca Degertekin C., Aktas Yilmaz B., Balos Toruner F., Kalkanci A., Turhan Iyidir O., Fidan I., Yesilyurt E., Cakır N., Kustimur S., Arslan M. Circulating Th17 Cytokine Levels Are Altered in Hashimoto’s Thyroiditis. Cytokine. 2016;80:13–17. doi: 10.1016/j.cyto.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Rydzewska M., Jaromin M., Pasierowska I.E., Stożek K., Bossowski A. Role of the T and B Lymphocytes in Pathogenesis of Autoimmune Thyroid Diseases. Thyroid Res. 2018;11:2. doi: 10.1186/s13044-018-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto E.A., Nguyen J.K., Liu J., Keller E., Campbell N., Zhang C.-J., Smith H.R., Li X., Jørgensen T.N. Low Levels of Vitamin D Promote Memory B Cells in Lupus. Nutrients. 2020;12:291. doi: 10.3390/nu12020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haas J., Schwarz A., Korporal-Kuhnke M., Faller S., Jarius S., Wildemann B. Hypovitaminosis D Upscales B-Cell Immunoreactivity in Multiple Sclerosis. J. Neuroimmunol. 2016;294:18–26. doi: 10.1016/j.jneuroim.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Chen S., Sims G.P., Chen X.X., Gu Y.Y., Chen S., Lipsky P.E. Modulatory Effects of 1,25-Dihydroxyvitamin D3 on Human B Cell Differentiation. J. Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J., Chen Y., Li H., Li H. Effects of Vitamin D on Thyroid Autoimmunity Markers in Hashimoto’s Thyroiditis: Systematic Review and Meta-Analysis. J. Int. Med. Res. 2021;49:03000605211060675. doi: 10.1177/03000605211060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J., Lv S., Chen G., Gao C., He J., Zhong H., Xu Y. Meta-Analysis of the Association between Vitamin D and Autoimmune Thyroid Disease. Nutrients. 2015;7:2485–2498. doi: 10.3390/nu7042485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Štefanić M., Tokić S. Serum 25-Hydoxyvitamin D Concentrations in Relation to Hashimoto’s Thyroiditis: A Systematic Review, Meta-Analysis and Meta-Regression of Observational Studies. Eur. J. Nutr. 2020;59:859–872. doi: 10.1007/s00394-019-01991-w. [DOI] [PubMed] [Google Scholar]
- 73.Lee G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018;19:730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li C., Yuan J., Zhu Y.-F., Yang X.-J., Wang Q., Xu J., He S.-T., Zhang J.-A. Imbalance of Th17/Treg in Different Subtypes of Autoimmune Thyroid Diseases. Cell. Physiol. Biochem. 2016;40:245–252. doi: 10.1159/000452541. [DOI] [PubMed] [Google Scholar]
- 75.Shao S., Yu X., Shen L. Autoimmune Thyroid Diseases and Th17/Treg Lymphocytes. Life Sci. 2018;192:160–165. doi: 10.1016/j.lfs.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 76.Zhou L., Wang J., Li J., Li T., Chen Y., June R.R., Zheng S.G. 1,25-Dihydroxyvitamin D3 Ameliorates Collagen-Induced Arthritis via Suppression of Th17 Cells Through MiR-124 Mediated Inhibition of IL-6 Signaling. Front. Immunol. 2019;10:178. doi: 10.3389/fimmu.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dankers W., Davelaar N., van Hamburg J.P., van de Peppel J., Colin E.M., Lubberts E. Human Memory Th17 Cell Populations Change into Anti-Inflammatory Cells with Regulatory Capacity upon Exposure to Active Vitamin D. Front. Immunol. 2019;10:1504. doi: 10.3389/fimmu.2019.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang S.H., Chung Y., Dong C. Vitamin D Suppresses Th17 Cytokine Production by Inducing C/EBP Homologous Protein (CHOP) Expression. J. Biol. Chem. 2010;285:38751–38755. doi: 10.1074/jbc.C110.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cvek M., Kaličanin D., Barić A., Vuletić M., Gunjača I., Torlak Lovrić V., Škrabić V., Punda A., Boraska Perica V. Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank. Nutrients. 2021;13:2793. doi: 10.3390/nu13082793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanna H.W.Z., Rizzo C., Abdel Halim R.M., El Haddad H.E., Salam R., El-Sayed Abou-Youssef H. Vitamin D Status in Hashimoto’s Thyroiditis and Its Association with Vitamin D Receptor Genetic Variants. J. Steroid Biochem. Mol. Biol. 2021;212:105922. doi: 10.1016/j.jsbmb.2021.105922. [DOI] [PubMed] [Google Scholar]
- 81.Filipova L., Lazurova Z., Fulop P., Lazurova I. Vitamin D insufficiency is not associated with thyroid autoimmunity in Slovak women with Hashimoto’s disease. Bratisl. Lek. Listy. 2023;124:182–186. doi: 10.4149/BLL_2023_029. [DOI] [PubMed] [Google Scholar]
- 82.Kim D. Low Vitamin D Status Is Associated with Hypothyroid Hashimoto’s Thyroiditis. Hormones. 2016;15:385–393. doi: 10.14310/horm.2002.1681. [DOI] [PubMed] [Google Scholar]
- 83.Kim M., Song E., Oh H.-S., Park S., Kwon H., Jeon M.J., Kim W.G., Kim W.B., Shong Y.K., Kim T.Y. Vitamin D Deficiency Affects Thyroid Autoimmunity and Dysfunction in Iodine-Replete Area: Korea National Health and Nutrition Examination Survey. Endocrine. 2017;58:332–339. doi: 10.1007/s12020-017-1425-z. [DOI] [PubMed] [Google Scholar]
- 84.Evliyaoğlu O., Acar M., Özcabı B., Erginöz E., Bucak F., Ercan O., Kucur M. Vitamin D Deficiency and Hashimoto’s Thyroiditis in Children and Adolescents: A Critical Vitamin D Level for This Association? J. Clin. Res. Pediatr. Endocrinol. 2015;7:128–133. doi: 10.4274/jcrpe.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Pergola G., Triggiani V., Bartolomeo N., Giagulli V.A., Anelli M., Masiello M., Candita V., De Bellis D., Silvestris F. Low 25 Hydroxyvitamin D Levels Are Independently Associated with Autoimmune Thyroiditis in a Cohort of Apparently Healthy Overweight and Obese Subjects. Endocr. Metab. Immune Disord. Drug Targets. 2018;18:646–652. doi: 10.2174/1871530318666180406163426. [DOI] [PubMed] [Google Scholar]
- 86.Xu J., Zhu X.-Y., Sun H., Xu X.-Q., Xu S.-A., Suo Y., Cao L.-J., Zhou Q., Yu H.-J., Cao W.-Z. Low Vitamin D Levels Are Associated with Cognitive Impairment in Patients with Hashimoto Thyroiditis. BMC Endocr. Disord. 2018;18:87. doi: 10.1186/s12902-018-0314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aktaş H.Ş. Vitamin B12 and Vitamin D Levels in Patients with Autoimmune Hypothyroidism and Their Correlation with Anti-Thyroid Peroxidase Antibodies. Med. Princ. Pract. 2020;29:364–370. doi: 10.1159/000505094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bozkurt N.C., Karbek B., Ucan B., Sahin M., Cakal E., Ozbek M., Delibasi T. The Association between Severity of Vitamin D Deficiency and Hashimoto’s Thyroiditis. Endocr. Pract. 2013;19:479–484. doi: 10.4158/EP12376.OR. [DOI] [PubMed] [Google Scholar]
- 89.Sayki Arslan M., Topaloglu O., Ucan B., Karakose M., Karbek B., Tutal E., Caliskan M., Ginis Z., Cakal E., Sahin M., et al. Isolated Vitamin D Deficiency Is Not Associated with Nonthyroidal Illness Syndrome, but with Thyroid Autoimmunity. Sci. World J. 2015;2015:239815. doi: 10.1155/2015/239815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Botelho I.M.B., Moura Neto A., Silva C.A., Tambascia M.A., Alegre S.M., Zantut-Wittmann D.E. Vitamin D in Hashimoto’s Thyroiditis and Its Relationship with Thyroid Function and Inflammatory Status. Endocr. J. 2018;65:1029–1037. doi: 10.1507/endocrj.EJ18-0166. [DOI] [PubMed] [Google Scholar]
- 91.Korzeniowska K., Jarosz-Chobot P., Szypowska A., Ramotowska A., Fendler W., Kalina-Faska B., Szadkowska A., Mlynarski W., Mysliwiec M. L-Thyroxine Stabilizes Autoimmune Inflammatory Process in Euthyroid Nongoitrous Children with Hashimoto’s Thyroiditis and Type 1 Diabetes Mellitus. J. Clin. Res. Pediatr. Endocrinol. 2013;5:240–244. doi: 10.4274/Jcrpe.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ke W., Sun T., Zhang Y., He L., Wu Q., Liu J., Zham B. 25-Hydroxyvitamin D serum level in Hashimoto’s thyroiditis, but not Graves’ disease is relatively deficient. Endocr. J. 2017;64:581–587. doi: 10.1507/endocrj.EJ16-0547. [DOI] [PubMed] [Google Scholar]
- 93.Feng Y., Qiu T., Chen H., Wei Y., Jiang X., Zhang H., Chen D. Association of Serum IL-21 and Vitamin D Concentrations in Chinese Children with Autoimmune Thyroid Disease. Clin. Chim. Acta. 2020;507:194–198. doi: 10.1016/j.cca.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 94.Hisbiyah Y., Endaryanto A., Setyoboedi B., Rochmah N., Faizi M. The Correlation between Vitamin D and Levels of IFN-γ, NF-ΚB, Thyroid Antibodies in down Syndrome: Study in Indonesian Children. Acta Biomed. 2022;93:e2022342. doi: 10.23750/abm.v93i6.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giuliani C., Bucci I., Napolitano G. The Role of the Transcription Factor Nuclear Factor-Kappa B in Thyroid Autoimmunity and Cancer. Front. Endocrinol. 2018;9:471. doi: 10.3389/fendo.2018.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roehlen N., Doering C., Hansmann M.-L., Gruenwald F., Vorlaender C., Bechstein W.O., Holzer K., Badenhoop K., Penna-Martinez M. Vitamin D, FOXO3a, and Sirtuin1 in Hashimoto’s Thyroiditis and Differentiated Thyroid Cancer. Front. Endocrinol. 2018;9:527. doi: 10.3389/fendo.2018.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ji J.S., Liu L., Shu C., Yan L.L., Zeng Y. Sex Difference and Interaction of SIRT1 and FOXO3 Candidate Longevity Genes on Life Expectancy: A 10-Year Prospective Longitudinal Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021;77:1557–1563. doi: 10.1093/gerona/glab378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nho R.S., Hergert P. FoxO3a and Disease Progression. World J. Biol. Chem. 2014;5:346–354. doi: 10.4331/wjbc.v5.i3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen C., Zhou M., Ge Y., Wang X. SIRT1 and Aging Related Signaling Pathways. Mech. Ageing Dev. 2020;187:111215. doi: 10.1016/j.mad.2020.111215. [DOI] [PubMed] [Google Scholar]
- 100.Tokić S., Štefanić M., Karner I., Glavaš-Obrovac L. Altered Expression of CTLA-4, CD28, VDR, and CD45 MRNA in T Cells of Patients with Hashimoto’s Thyroiditis—A Pilot Study. Endokrynol. Pol. 2017;68:274–828. doi: 10.5603/EP.2017.0020. [DOI] [PubMed] [Google Scholar]
- 101.Krysiak R., Kowalcze K., Okopień B. The Impact of Exogenous Vitamin D on Thyroid Autoimmunity in Euthyroid Men with Autoimmune Thyroiditis and Early-Onset Androgenic Alopecia. Pharmacol. Rep. 2021;73:1439–1447. doi: 10.1007/s43440-021-00295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krysiak R., Kowalcze K., Okopień B. The Effect of Vitamin D on Thyroid Autoimmunity in Euthyroid Men with Autoimmune Thyroiditis and Testosterone Deficiency. Pharmacol. Rep. 2019;71:798–803. doi: 10.1016/j.pharep.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 103.Krysiak R., Kowalcze K., Okopień B. Hyperprolactinaemia Attenuates the Inhibitory Effect of Vitamin D/Selenomethionine Combination Therapy on Thyroid Autoimmunity in Euthyroid Women with Hashimoto’s Thyroiditis: A Pilot Study. J. Clin. Pharm. Ther. 2020;45:1334–1341. doi: 10.1111/jcpt.13214. [DOI] [PubMed] [Google Scholar]
- 104.Pludowski P., Takacs I., Boyanov M., Belaya Z., Diaconu C.C., Mokhort T., Zherdova N., Rasa I., Payer J., Pilz S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients. 2022;14:1483. doi: 10.3390/nu14071483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Płudowski P., Kos-Kudła B., Walczak M., Fal A., Zozulińska-Ziółkiewicz D., Sieroszewski P., Peregud-Pogorzelski J., Lauterbach R., Targowski T., Lewiński A., et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients. 2023;15:695. doi: 10.3390/nu15030695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krysiak R., Szkróbka W., Okopień B. Dehydroepiandrosterone Potentiates the Effect of Vitamin D on Thyroid Autoimmunity in Euthyroid Women with Autoimmune Thyroiditis: A Pilot Study. Clin. Exp. Pharmacol. Physiol. 2021;48:195–202. doi: 10.1111/1440-1681.13410. [DOI] [PubMed] [Google Scholar]
- 107.Krysiak R., Szkróbka W., Okopień B. Moderate-Dose Simvastatin Therapy Potentiates the Effect of Vitamin D on Thyroid Autoimmunity in Levothyroxine-Treated Women with Hashimoto’s Thyroiditis and Vitamin D Insufficiency. Pharmacol. Rep. 2018;70:93–97. doi: 10.1016/j.pharep.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 108.Krysiak R., Kowalcze K., Okopień B. Selenomethionine Potentiates the Impact of Vitamin D on Thyroid Autoimmunity in Euthyroid Women with Hashimoto’s Thyroiditis and Low Vitamin D Status. Pharmacol. Rep. 2019;71:367–373. doi: 10.1016/j.pharep.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 109.Krysiak R., Kowalcze K., Okopien B. Gluten-Free Diet Attenuates the Impact of Exogenous Vitamin D on Thyroid Autoimmunity in Young Women with Autoimmune Thyroiditis: A Pilot Study. Scand. J. Clin. Lab. Investig. 2022;82:518–524. doi: 10.1080/00365513.2022.2129434. [DOI] [PubMed] [Google Scholar]
- 110.Malandrini S., Trimboli P., Guzzaloni G., Virili C., Lucchini B. What about TSH and Antithyroid Antibodies in Patients with Autoimmune Thyroiditis and Celiac Disease Using a Gluten-Free Diet? A Systematic Review. Nutrients. 2022;14:1681. doi: 10.3390/nu14081681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pobłocki J., Pańka T., Szczuko M., Telesiński A., Syrenicz A. Whether a Gluten-Free Diet Should Be Recommended in Chronic Autoimmune Thyroiditis or Not?—A 12-Month Follow-Up. J. Clin. Med. 2021;10:3240. doi: 10.3390/jcm10153240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mazokopakis E.E., Papadomanolaki M.G., Tsekouras K.C., Evangelopoulos A.D., Kotsiris D.A., Tzortzinis A.A. Is Vitamin D Related to Pathogenesis and Treatment of Hashimoto’s Thyroiditis? Hell. J. Nucl. Med. 2015;18:222–227. [PubMed] [Google Scholar]
- 113.Chaudhary S., Dutta D., Kumar M., Saha S., Mondal S.A., Kumar A., Mukhopadhyay S. Vitamin D Supplementation Reduces Thyroid Peroxidase Antibody Levels in Patients with Autoimmune Thyroid Disease: An Open-Labeled Randomized Controlled Trial. Indian J. Endocrinol. Metab. 2016;20:391. doi: 10.4103/2230-8210.179997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aghili A., Alijanpour Aghamaleki M., Pornasrollah M., Nooreddini H.G., Khafri S., Alijanpour S. Effect of Vitamin D Therapy on Hashimoto’s Thyroiditis in Children with Hypovitaminosis D. Int. J. Pediatr. 2020;8:10889–10897. doi: 10.22038/ijp.2019.42711.3579. [DOI] [Google Scholar]
- 115.Simsek Y., Cakir I., Yetmis M., Dizdar O.S., Baspinar O., Gokay F. Effects of Vitamin D Treatment on Thyroid Autoimmunity. J. Res. Med. Sci. 2016;21:85. doi: 10.4103/1735-1995.192501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mirhosseini N., Brunel L., Muscogiuri G., Kimball S. Physiological Serum 25-Hydroxyvitamin D Concentrations Are Associated with Improved Thyroid Function—Observations from a Community-Based Program. Endocrine. 2017;58:563–573. doi: 10.1007/s12020-017-1450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vondra K., Bilek R., Matucha P., Salatova M., Vosatkova M., Starka L., Hampl R. Vitamin D Supplementation Changed Relationships, Not Levels of Metabolic-Hormonal Parameters in Autoimmune Thyroiditis. Physiol. Res. 2017;66:S409–S417. doi: 10.33549/physiolres.933727. [DOI] [PubMed] [Google Scholar]
- 118.Behera K.K., Saharia G.K., Hota D., Sahoo D.P., Sethy M., Srinivasan A. Effect of Vitamin D Supplementation on Thyroid Autoimmunity among Subjects of Autoimmune Thyroid Disease in a Coastal Province of India: A Randomized Open-Label Trial. Niger. Med. J. 2020;61:237. doi: 10.4103/nmj.NMJ_200_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robat-Jazi B., Mobini S., Chahardoli R., Mansouri F., Nodehi M., Esfahanian F., Saboor Yaraghi A.A. The Impact of Vitamin D Supplementation on the IFNγ-IP10 Axis in Women with Hashimoto’s Thyroiditis Treated with Levothyroxine: A Double-blind Randomized Placebo-controlled Trial. Iran. J. Allergy Asthma Immunol. 2022;21:407–417. doi: 10.18502/ijaai.v21i4.10288. [DOI] [PubMed] [Google Scholar]
- 120.Karin N., Razon H. Chemokines beyond Chemo-Attraction: CXCL10 and Its Significant Role in Cancer and Autoimmunity. Cytokine. 2018;109:24–28. doi: 10.1016/j.cyto.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 121.Antonelli A., Ferrari S.M., Giuggioli D., Ferrannini E., Ferri C., Fallahi P. Chemokine (C-X-C Motif) Ligand (CXCL)10 in Autoimmune Diseases. Autoimmun. Rev. 2014;13:272–280. doi: 10.1016/j.autrev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 122.Nodehi M., Ajami A., Izad M., Omran H.A., Chahardoli R., Amouzegar A., Yekaninejad S., Hemmatabadi M., Azizi F., Esfahanian F., et al. Effects of Vitamin D Supplements on Frequency of CD4+ T-Cell Subsets in Women with Hashimoto’s Thyroiditis: A Double-Blind Placebo-Controlled Study. Eur. J. Clin. Nutr. 2019;73:1236–1243. doi: 10.1038/s41430-019-0395-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.