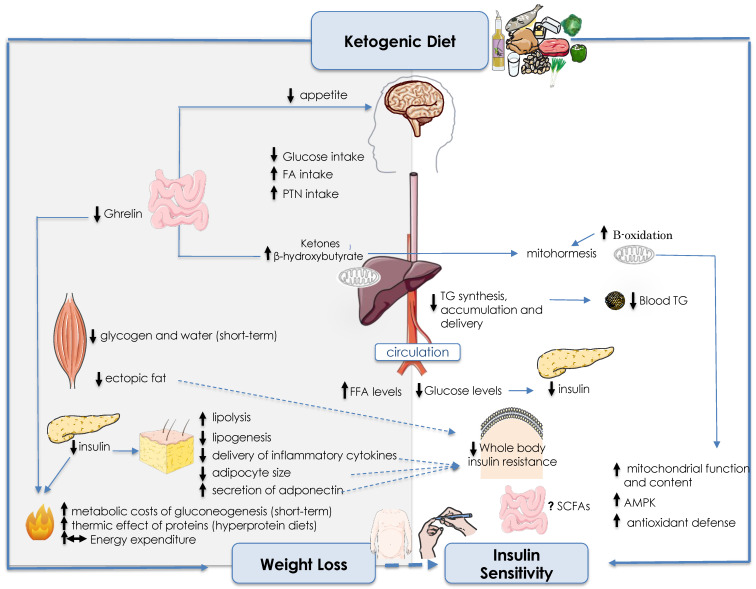
Figure 1.
Summary of suggested mechanisms of ketogenic diets on weight loss (left) and insulin sensitivity (right). The severe restriction in digestible carbohydrates’ intake reduces insulin secretion leading to decreased lipogenesis, and increased lipolysis and blood levels of ketone bodies (KBs). Ketone bodies may reduce appetite—and consequently energy intake—directly (via mechanisms not yet elucidated) or indirectly (via hormones). The increased rate of lipolysis contributes to weight loss by reducing fat deposits (in many tissues) and adipocyte size, leading to a decreased delivery of inflammatory cytokines and increased secretion of adiponectin, that, in turn, improve whole body insulin signaling. The low availability of dietary digestible carbohydrates also decreases hepatic glycogen, de novo synthesis, and delivery of triglycerides (TG) from hepatocytes, improving liver insulin sensitivity and blood lipoprotein profile independently of weight loss. Additionally, the increased TG hydrolysis toward β-oxidation and subsequently ketogenesis enhance mitochondrial function and resistance to oxidative stress (known as mitohormesis), improving insulin signaling. There is conflicting evidence regarding the effects of KDs on energy expenditure and short-chain fatty acids (SCFA) production by gut microbiota, which is dependent on many aspects, such as the content of dietary protein, fiber, probiotics, etc. Dotted lines indicate the mediated effects of weight loss on insulin sensitivity. Abbreviations: Fatty acids (FA); Protein (PTN); Free fatty acids (FFAs); Adenosine monophosphate-activated protein kinase (AMPK).