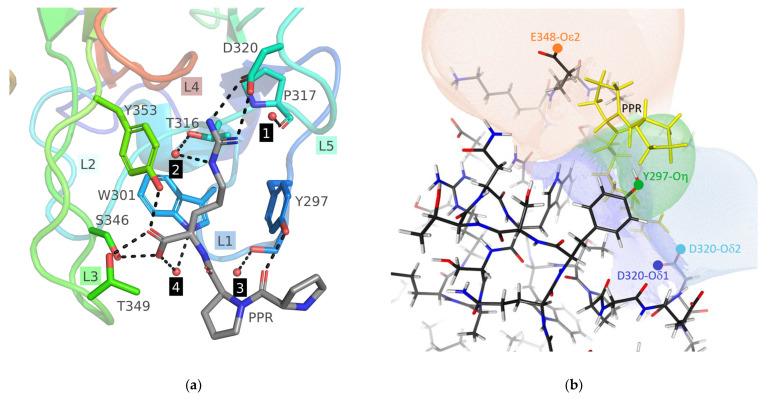
Figure 3.
(a) Structure of the binding site of NRP1-b1 hexavariant in complex with the KDKPPR peptide. The pocket is mainly composed of five loops (L1 to L5), which are respectively colored blue, cyan, green, red and turquoise. The crystallographic model of the peptide includes only the PPR moiety, represented as sticks. The NRP1 residues in the close proximity of the peptide are also depicted as sticks. The four structural water molecules are highlighted as spheres, while hydrogen bonds are illustrated as dashed sticks. Various labels are provided to enhance clarity, indicating loops, peptide, residues and water molecules. (b) Nucleophilic Influence Zones associated with the oxygen atoms Asp320-Oδ1 (dark blue), Asp320-Oε2 (light blue), Tyr297-Oη (green) and Glu348-Oε2 (major conformer, orange) in the vicinity of the ligand-binding site of monomer A. The corresponding atomic nucleophilic sites are indicated by colored circles, and the PPR moiety of the KDKPPR peptide is highlighted in yellow.