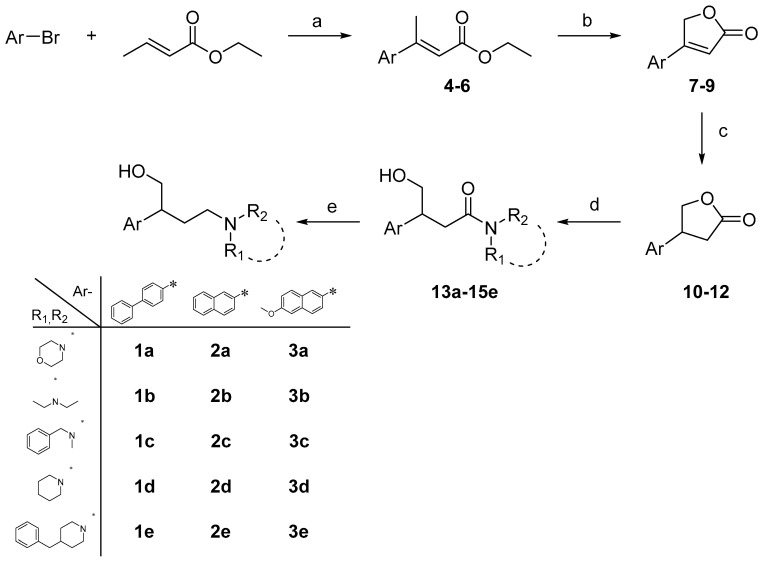
Scheme 1.
Reagents and conditions: (a) 4-phenyl-bromo-benzene (for 4, 7, 10, and 13), 2-bromo-naphtalene (for 5, 8, 11, and 14), or 5-methoxy-bromo-naphtalene (for 6, 9, 12, and 15) (1 equiv.), ethyl crotonate (1.5 equiv.), AcOEt (2 equiv.), Bu4NCl (2 equiv.), and Pd(OAc)2 (0.05 equiv.), anh. DMF, N2, 105 °C, 3–5 h, 51–59% yield; (b) 4–6 (1 equiv.), SeO2 (1.3 equiv.), CH3CN, mw T = 100 °C, W = 180, PSI = 200, run time = 15 min each cycle; (c) 7–9 (1 equiv.) HCO2NH4 (5 equiv.), Pd/C (35% wt), AcOEt/t-BuOH, mw T = 100 °C, W = 180, PSI = 200, run time = 5 min, 31–53% yield over two steps; (d) morpholine (for 13a–15a), diethylamine (for 13b–15b), N-benzyl-N-methylamine (for 13c–15c), piperidine (for 13d–15d), 4-benzylpiperidine (for 13e–15e) (2.5 equiv.), AlCl3 (1.3 equiv.), 10–12 (1 equiv.), 1,2-dichloroetane, 0° C to r.t., 3–5 h, 40–99% yield; e) 13a–15e (1 equiv.), LiAlH4 (2 equiv.), anh. THF, −15 °C to r.t., 2–4 h, 13–67% yield.