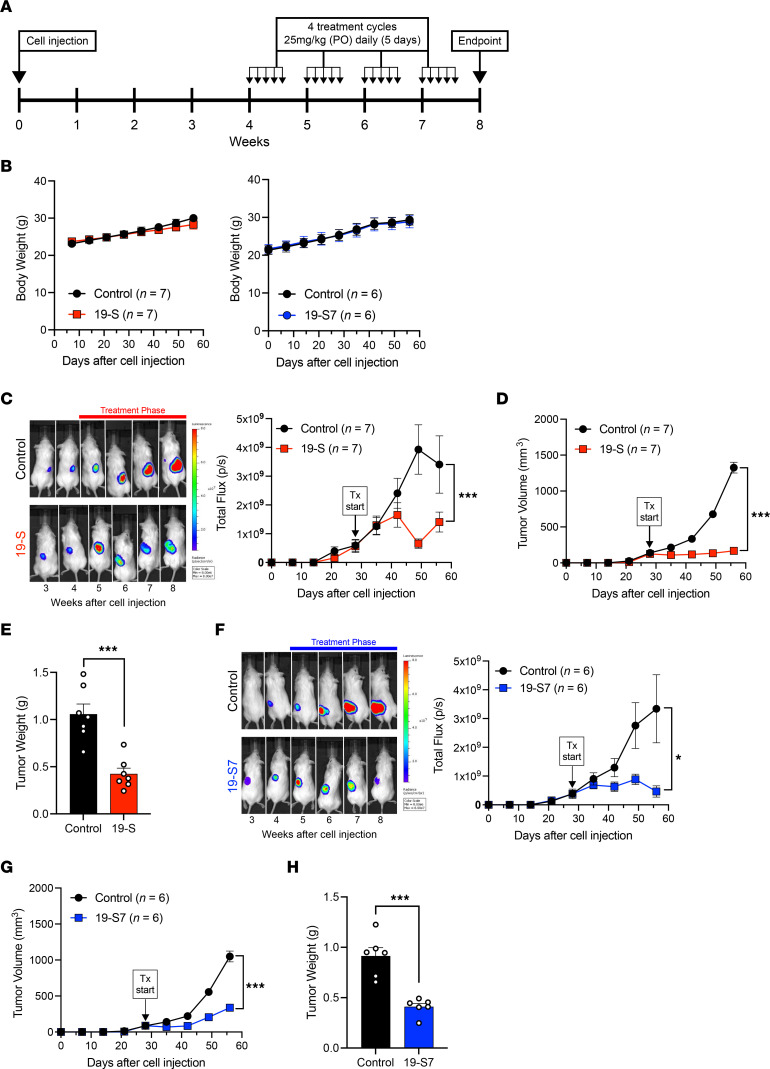
Figure 9. Compounds 19-S and 19-S7 prevent tumor progression without signs of toxicity following oral delivery.
(A) Timeline indicating the subcutaneous injection of Luc-PANC-1 cells to generate subcutaneous tumors and the treatment cycles for compound 19-S (25 mg/kg), compound 19-S7 (25 mg/kg), or vehicle control administered PO. For each cycle animals were treated once a day for 5 continuous days, then allowed 2 days’ rest when no treatments were administered. NSG mice received 4 treatment cycles until endpoint. (B) Effect of compound 19-S and 19-S7 treatment with matched vehicle control treatment on body weight for Luc-PANC-1 tumor–bearing NSG mice. Data are expressed as mean ± SEM of compound 19-S (n = 7), 19-S7 (n = 6), and controls (n = 7 and n = 6, respectively). (C) Bioluminescence imaging of Luc-PANC-1–derived tumors and quantification of bioluminescence photon flux over time for animals treated PO with compound 19-S or vehicle control (***P = 0.0002). (D) Luc-PANC-1 tumor volumes for animals treated with compound 19-S or vehicle control administered PO (***P < 0.0001). (E) Final excised Luc-PANC-1 tumor weight for animals treated PO with compound 19-S or vehicle control (***P = 0.0010). (F) Bioluminescence imaging of Luc-PANC-1 derived tumors and quantification of bioluminescence photon flux over time for animals treated PO with compound 19-S7 or vehicle control (*P = 0.0192). (G) Luc-PANC-1 tumor volumes for animals treated PO with compound 19-S7 or vehicle control (***P < 0.0001). (H) Final excised Luc-PANC-1 tumor weight for animals treated PO with compound 19-S7 or vehicle control, ***P = 0.0010. Data are expressed as mean ± SEM of n = 7 for 19-S and n = 6 for 19-S7 treatment. Statistical analysis in C, D, F, and G was performed using 2-way ANOVA; in E and H unpaired t test with Welch’s correction was used.