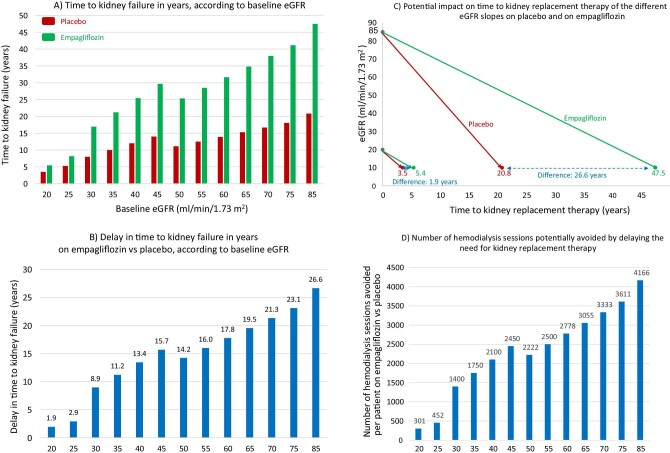
Figure 3:
Hypothetical transformation of chronic eGFR slopes into time to kidney failure, defined as eGFR 10 mL/min/1.73 m2, in the EMPA-KIDNEY trial. (A) Time to kidney failure in years, according to baseline eGFR, estimated from each baseline eGFR value by applying the chronic eGFR slopes corresponding to participants on placebo and on empagliflozin within the pre-specified eGFR subgroups (eGFR cut-off points to define subgroups set at 30 and 45 mL/min/1.73 m2) as per reference [9]. (B) Delay in time (years) to kidney failure on empagliflozin vs placebo, according to baseline eGFR, obtained by subtracting the time to kidney failure on empagliflozin from the time to kidney failure on placebo in (A). (C) Graphical presentation of representative chronic eGFR slopes from baseline to kidney failure, i.e. to the need for kidney replacement therapy. Hypothetical lines have been traced starting from extremes of the baseline eGFR inclusion criteria values (20 and 85 mL/min/1.73 m2) to eGFR 10 mL/min/1.73 m2, corresponding to chronic eGFR slopes of participants on placebo and on empagliflozin within each baseline eGFR subgroup, as per reference [9]. The difference in the time to kidney failure corresponds to the values in (B) for baseline 20 and 85 mL/min/1.73 m2. (D) Number of hemodialysis sessions potentially avoided by delaying the need for kidney replacement therapy by prescribing empagliflozin instead of placebo at each baseline eGFR value. The model assumes that patients will live up to the point where they need kidney replacement therapy and that they would continue hemodialysis throughout. While this is not expected to occur in every patient, it is a real possibility for some of them.