ABSTRACT
In this issue of Clinical Kidney Journal, Stehlé and colleagues demonstrate that estimation of glomerular filtration rate (GFR) by use of creatinine and a measure, total lumbar muscle cross-sectional area, reflecting the total muscle mass of an individual, is superior to GFR-estimating equations based upon creatinine and demographic variables. The report by Stehlé et al. demonstrates one solution to the interference of muscle mass in the use of creatinine to estimate GFR. This interference was identified already at the start, in 1959, of using creatinine for estimation of GFR. Different ways of taking the muscle mass into account when creatinine-based estimations of GFR have been used generally include use of controversial race and sex coefficients. A new marker of GFR, cystatin C, introduced in 1979, has been shown to be virtually uninfluenced by muscle mass. In this editorial, the simultaneous use of creatinine and cystatin C to estimate GFR, muscle mass and selective glomerular hypofiltration syndromes is described.
Keywords: creatinine, cystatin C, sarcopenia, selective glomerular hypofiltration, shrunken pore syndrome
Graphical Abstract
Graphical Abstract.
CREATININE, MUSCLE MASS, SEX AND RACE COEFFICIENTS
The introduction of endogenous creatinine clearance as a measure of glomerular filtration rate (GFR) by Rehberg in 1926 [1] developed in 1959 and 1971 into using plasma or serum creatinine for estimation of GFR [2, 3] and, later, to the use of more complex creatinine-based GFR-estimating equations [4]. However, even the first reports suggesting serum creatinine as a potential marker of GFR observed that increasing muscle mass produced increasing serum levels of creatinine and that a sex coefficient had to be used to estimate GFR in males and females due to the fact that the average muscle mass in males is 10%–25% bigger than in females [2, 3]. Indeed, some investigations also show that for healthy people with normal measured GFR (iohexol clearance), the creatinine level is significantly correlated to the measured muscle mass (dual-energy X-ray absorptiometry, DEXA) of the individuals, but not to their GFR [5]. It was also noted that different populations display different average muscle mass and in 1999 the first creatinine-based GFR estimating equation using race coefficients was described [6]. Since then, at least 10 different race coefficients have been used in creatinine-based GFR-estimating equations [7]. This has been an unfortunate development as ‘race’ cannot be determined by biological measurements [8–11], and usually is based upon self-reporting [12]. The concept of race is therefore subjective and more associated with sociological circumstances than objective biological facts [12]. It is also associated with discomfort of the self-reporting individual, and misclassification may lead to reduced access to care [12]. Therefore, strong recommendations have recently been issued that race coefficients should not be used in creatinine-based GFR-estimating equations [7, 13–15], and a new creatinine-based GFR-estimating equation without a race coefficient has recently been described [16]. As expected, its diagnostic performance was reduced compared with the corresponding equation with a race coefficient [16]. Another way of avoiding race coefficients in creatinine-based GFR-estimating equations is to measure total lumbar muscle cross-sectional area by unenhanced CT scan at the third lumbar vertebra and include this value in the GFR-estimating equation, as described by Stehlé et al. in this issue of Clinical Kidney Journal [17].
Although use of race coefficients in creatinine-based equations is problematic and should be avoided, the use of sex coefficients might also be problematic [7]. Although it for many years has been assumed that the sex of a person always is a binary parameter, recent research has demonstrated that this is not the optimal description of reality [18–20]. The construction of creatinine-based GFR-estimating equations has previously always assumed that sex is a binary parameter, but today several nations, e.g. Germany, the USA, Australia, Canada, India, Sweden and Denmark, have applied recent research and specify more than two genders in basic descriptions of their citizens [21]. Self-reporting of sex is often used in healthcare and if only two alternatives are allowed, a significant proportion of the patients will experience discomfort connected to the acknowledgment of only two genders in national legislations and other issues pertaining to the LGBTQIA+ spectrum [22, 23]. An additional problem connected to self-reporting of sex is that a change in the self-reported sex results in a major change in creatinine-based estimations of GFR [24]. These problems associated with the use of sex coefficients will be eliminated by use of the GFR-estimating equation described by Stehlé et al. in this issue of Clinical Kidney Journal [17]. However, there may be an easier way to avoid confounding from muscle mass.
CYSTATIN C, MUSCLE MASS, SEX AND RACE COEFFICIENTS
Already in the initial studies of the relationship between GFR and cystatin C, it was shown that no sex coefficient was required to obtain a high correlation between GFR and cystatin C [25], in contrast to when creatinine was used [25]. This suggested that differences in muscle mass between individuals did not interfere in cystatin C–based estimation of GFR, meaning that no sex or race coefficients would be required in cystatin C–based GFR-estimating equations. Indeed, several recent studies of cystatin C and GFR have demonstrated that neither race nor sex coefficients are required in cystatin C–based GFR-estimating equations [7, 26–29]. Therefore, use of cystatin C–based GFR-estimating equations is an easy option for avoiding the use of race or sex coefficients in estimation of GFR.
ANALYSING MUSCLE MASS AND SARCOPENIA
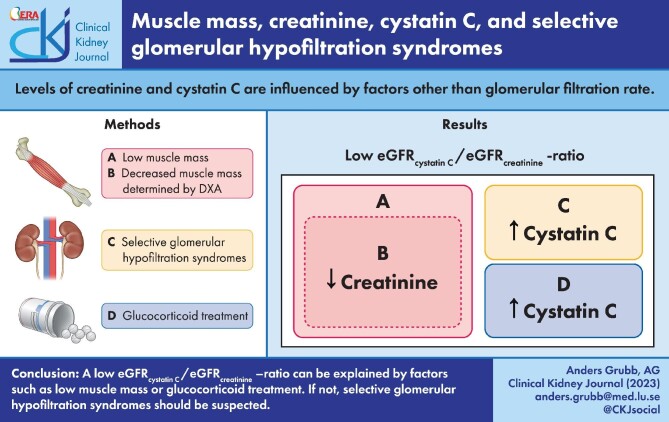
Even if the measurement of muscle mass and using this parameter in creatinine-based GFR-estimating equations, as described by Stehlé et al. in this issue of Clinical Kidney Journal, allows omission of race and sex variables in such equations [17], reliable measurement of muscle mass is technically difficult and time consuming [5, 17]. The diagnosis of sarcopenia, a state of both low muscle mass and low strength, requires the measurement of appendicular skeletal muscle mass, for example using DEXA and grip strength to estimate muscle strength [30]. Since creatinine, in contrast to cystatin C, is strongly related to the muscle mass of a person and both analytes are related to GFR, easier and less expensive ways have been suggested to estimate the muscle mass status of a person. One example of this is the Sarcopenia Index [(serum creatinine/cystatin C) × 100], recently suggested to be useful for monitoring musculoskeletal status in drug trials aiming to treat sarcopenia [31]. Ratios between cystatin C– and creatinine-based GFR estimates have also been used to estimate muscle mass and a eGFRcystatin C/eGFRcreatinine ratio below about 0.90 has in many studies been found to indicate a low muscle mass [5, 32–41].
Identifying individuals suffering from sarcopenia is important, since it is a key component of frailty, a state of functional decline in multiple organ systems [41, 42].
IDENTIFYING SELECTIVE GLOMERULAR HYPOFILTRATION SYNDROMES
Although a low eGFRcystatin C/eGFRcreatinine ratio usually indicates a low muscle mass, it might also identify another group of syndromes called selective glomerular hypofiltration syndromes first described in 2015 [43–48]. In these syndromes, e.g. shrunken and elongated pore syndromes, the glomerular filtration of 5–30 kDa molecules, such as cystatin C, is selectively reduced compared with the filtration of small molecules <1 kDa dominating the glomerular filtrate, e.g. water, creatinine and urea [44, 45]. Although the changes in the curves relating sieving coefficients to molecular mass of the filtered molecules in these syndromes usually are obvious from the changes in the resulting proteomes, a few invasive studies showing the same phenomena have also been performed, especially in pregnancy [49]. In the third trimester of all pregnancies the filtration of 5–30 kDa molecules is selectively reduced, but a few weeks after birth the filtration curves are normalized [44, 45]. Selective glomerular hypofiltration syndromes display a prevalence between 0.3% and 36% in different populations [44, 45] and are strongly connected to increases in mortality or morbidity [44–48]. The pathophysiological mechanism explaining the increase in mortality and morbidity in these conditions is probably connected to the altered proteomes [44, 45]. Whether a decreased eGFRcystatin C/eGFRcreatinine ratio indicates sarcopenia or selective glomerular hypofiltration syndromes is often obvious from clinical observations on muscle weakness, low body mass index and progressive muscle wasting, but in some cases measuring muscle mass might be required, e.g. as described by Stehlé et al. [17].
DIAGNOSTIC ALTERNATIVES OF A LOW eGFRcystatin C/eGFRcreatinine RATIO
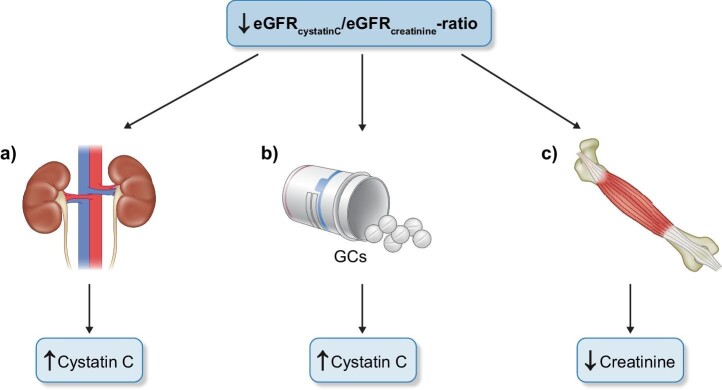
The two diagnostic interpretations of a low eGFRcystatin C/eGFRcreatinine ratio given above, i.e. sarcopenia and selective glomerular hypofiltration syndromes, must be complemented by the knowledge that treatment of patients with moderate to high doses of corticosteroids will induce an increase in the production of cystatin C, thereby producing a low eGFRcystatin C/eGFRcreatinine ratio unrelated to the diagnoses mentioned above [44, 45, 50]. However, in clinical practice the differentiation between these diagnostic alternatives will most often be simple and only rarely require measurement of muscle mass, e.g. as suggested by Stehlé et al. in this issue of Clinical Kidney Journal [17]. Figure 1 shows the dominating conditions indicated by a low eGFRcystatin C/eGFRcreatinine ratio.
Figure 1:
A low eGFRcystatin C/eGFRcreatinine ratio usually indicates a) selective glomerular hypofiltration disorders e.g., shrunken pore or elongated pore syndromes, b) glucocorticoid treatment or c) sarcopenia.
Contributor Information
Linnea Malmgren, Department of Clinical Sciences Malmö, Clinical and Molecular Osteoporosis Research Unit, Lund University, Malmö, Sweden; Department of Geriatrics, Skåne University Hospital, Malmö, Sweden.
Anders Grubb, Department of Clinical Chemistry, Skåne University Hospital, Lund University, Lund, Sweden.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Rehberg PB. Studies on kidney function. The rate of filtration and reabsorption in the human kidney. Biochem J 1926;20:447–60. 10.1042/bj0200447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edwards KD, Whyte HM. Plasma creatinine level and creatinine clearance as tests of renal function. Australas Ann Med 1959;8:218–24. 10.1111/imj.1959.8.3.218. [DOI] [PubMed] [Google Scholar]
- 3. Jelliffe R, Jelliffe S. Estimation of creatinine clearance from changing serum-creatinine levels. Lancet North Am Ed 1971;298:710. 10.1016/S0140-6736(71)92283-5. [DOI] [PubMed] [Google Scholar]
- 4. Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis 2014;63:820–34. 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vinge E, Lindergård B, Nilsson-Ehle Pet al. . Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 1999;59:587–92. 10.1080/00365519950185076. [DOI] [PubMed] [Google Scholar]
- 6. Levey AS, Bosch JP, Lewis JBet al. . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 7. Ottosson Frost C, Gille-Johnson P, Blomstrand Eet al. . Cystatin C-based equations for estimating glomerular filtration rate do not require race or sex coefficients. Scand J Clin Lab Invest 2022;82:162–6. 10.1080/00365513.2022.2031279. [DOI] [PubMed] [Google Scholar]
- 8. Fontanarosa PB, Bauchner H. Race, ancestry, and medical research. JAMA 2018;320:1539–40. 10.1001/jama.2018.14438. [DOI] [PubMed] [Google Scholar]
- 9. Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA 2019;322:113–4. 10.1001/jama.2019.5774. [DOI] [PubMed] [Google Scholar]
- 10. Powe NR. Black kidney function matters: use or misuse of race? JAMA 2020;324:737–8. 10.1001/jama.2020.13378. [DOI] [PubMed] [Google Scholar]
- 11. Flanagin A, Frey T, Christiansen SLet al. . The reporting of race and ethnicity in medical and science journals: comments invited. JAMA 2021;325:1049–52. 10.1001/jama.2021.2104. [DOI] [PubMed] [Google Scholar]
- 12. Gama RM, Clery A, Griffiths Ket al. . Estimated glomerular filtration rate equations in people of self-reported black ethnicity in the United Kingdom: inappropriate adjustment for ethnicity may lead to reduced access to care. PLoS One 2021;16:e0255869. 10.1371/journal.pone.0255869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsu C, Yang W, Parikh RVet al. . Race, genetic ancestry, and estimating kidney function in CKD. N Engl J Med 2021;385:1750–60. 10.1056/NEJMoa2103753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inker LA, Eneanya ND, Coresh Jet al. . New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams WW, Hogan JW, Ingelfinger JR. Time to eliminate health care disparities in the estimation of kidney function. N Engl J Med 2021;385:1804–6. 10.1056/NEJMe2114918. [DOI] [PubMed] [Google Scholar]
- 16. Inker LA, Eneanya ND, Coresh Jet al. . Chronic kidney disease epidemiology collaboration. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stehlé T, Quamri Y, Fellahi Set al. . Development and validation of a new equation based on plasma creatinine and muscle mass assessed by CT-scan to estimate glomerular filtration rate: a cross-sectional study. Clin Kidney J 2023; In press. 10.1093/ckj/sfad012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ainsworth C. Sex redefined. The idea of two sexes is simplistic. Biologists now think there is a wider spectrum than that. Nature 2015;518:288–91. 10.1038/518288a. [DOI] [PubMed] [Google Scholar]
- 19. Hyde JS, Bigler RS, Joel Det al. . The future of sex and gender in psychology: five challenges to the gender binary. Am Psychol 2019;74:171–93. 10.1037/amp0000307. [DOI] [PubMed] [Google Scholar]
- 20. Joel D. Beyond the binary: rethinking sex and the brain. Neurosci Biobehav Rev 2021;122:165–75. 10.1016/j.neubiorev.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 21. Rogers K. CNN. How the letter X is changing the game for travelers—and what that could mean for the US. Available from: https://edition.cnn.com/travel/article/countries-with-third-gender-x-passports/index.html (5 February 2023, date last accessed).
- 22. Zucker KJ. Epidemiology of gender dysphoria and transgender identity. Sex Health 2017;14:404–11. 10.1071/SH17067. [DOI] [PubMed] [Google Scholar]
- 23. Logie CH, Lys CL, Dias Let al. “Automatic assumption of your gender, sexuality and sexual practices is also discrimination”: exploring sexual healthcare experiences and recommendations among sexually and gender diverse persons in Arctic Canada. Health Soc Care Community 2019;27:1204–13. [DOI] [PubMed] [Google Scholar]
- 24. Fernandez-Prado R, Ortiz A. A sudden decrease in serum creatinine and estimated glomerular filtration rate: clinical implications of administrative gender assignment in transgender persons. Clin Kidney J 2019;3:1107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grubb A, Simonsen O, Sturfelt Get al. . Serum concentration of cystatin C, factor D and β2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand 1985;218:499–503. 10.1111/j.0954-6820.1985.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 26. Grubb A, Björk J, Lindström Vet al. . A cystatin C-based formula without anthropometric variables estimates glomerular filtration rate better than creatinine clearance using the Cockcroft-Gault formula. Scand J Clin Lab Invest 2005;65:153–62. 10.1080/00365510510013596. [DOI] [PubMed] [Google Scholar]
- 27. Rule AD, Bergstralh EJ, Slezak JMet al. . Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int 2006;69:399–405. 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 28. Grubb A, Horio M, Hansson LOet al. . Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem 2014;60:974–86. 10.1373/clinchem.2013.220707. [DOI] [PubMed] [Google Scholar]
- 29. Pottel H, Björk J, Rule ADet al. . Cystatin C–based equation to estimate GFR without the inclusion of race and sex. N Engl J Med 2023;388:333–43. 10.1056/NEJMoa2203769. [DOI] [PubMed] [Google Scholar]
- 30. Cruz-Jentoft AJ, Bahat G, Bauer Jet al. . Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ladang A, Beaudart C, Reginster JYet al. . Biochemical markers of musculoskeletal health and aging to be assessed in clinical trials of drugs aiming at the treatment of sarcopenia: consensus paper from an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the Centre Académique de Recherche et d'Expérimentation en Santé (CARES SPRL), Under the Auspices of the World Health Organization Collaborating Center for the Epidemiology of Musculoskeletal Conditions and Aging. Calcif Tissue Int 2023;112:197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kashani KB, Frazee EN, Kukrálová Let al. . Evaluating muscle mass by using markers of kidney function. Crit Care Med 2017;45:e23–9. 10.1097/CCM.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 33. Yang J, Zhang T, Feng Det al. . A new diagnostic index for sarcopenia and its association with short-term postoperative complications in patients undergoing surgery for colorectal cancer. Colorectal Dis 2019;21:538–47. 10.1111/codi.14558. [DOI] [PubMed] [Google Scholar]
- 34. Nishida K, Hashimoto Y, Kaji Aet al. . Creatinine/(cystatin C × body weight) ratio is associated with skeletal muscle mass index. Endocr J 2020;67:733–40. 10.1507/endocrj.EJ19-0542. [DOI] [PubMed] [Google Scholar]
- 35. Lin YL, Chen SY, Lai YHet al. . Serum creatinine to cystatin C ratio predicts skeletal muscle mass and strength in patients with non-dialysis chronic kidney disease. Clin Nutr 2020;39:2435–41. 10.1016/j.clnu.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 36. Fu X, Tian Z, Wen Set al. . A new index based on serum creatinine and cystatin C is useful for assessing sarcopenia in patients with advanced cancer. Nutrition 2021;82:111032. 10.1016/j.nut.2020.111032. [DOI] [PubMed] [Google Scholar]
- 37. Rizk JG, Streja E, Wenziger Cet al. . Serum creatinine-to-cystatin-C ratio as a potential muscle mass surrogate and racial differences in mortality. J Ren Nutr 2023;33:69–77. 10.1053/j.jrn.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 38. Yoshida S, Nakayama Y, Nakayama Jet al. . Assessment of sarcopenia and malnutrition using estimated GFR ratio (eGFRcys/eGFR) in hospitalised adult patients. Clin Nutr ESPEN 2022;48:456–63. 10.1016/j.clnesp.2021.12.027. [DOI] [PubMed] [Google Scholar]
- 39. Tlemsani C, Durand J-P, Raynard Bet al. . Relationship between the creatinine/cystatin C ratio and muscle mass measured by CT-scan in cancer patients. Clin Nutr ESPEN 2022;51:412–8. 10.1016/j.clnesp.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 40. Kusunoki H, Tabara Y, Tsuji Set al. . Estimation of muscle mass using creatinine/cystatin C ratio in Japanese community-dwelling older people. J Am Med Dir Assoc 2022;23:902.e21–31. 10.1016/j.jamda.2021.07.029. [DOI] [PubMed] [Google Scholar]
- 41. Tang T, Xie L, Hu Set al. . Serum creatinine and cystatin C-based diagnostic indices for sarcopenia in advanced non-small cell lung cancer. J Cachexia Sarcopenia Muscle 2022;13:1800–10. 10.1002/jcsm.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001;1:323–36. 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grubb A, Lindström V, Jonsson Met al. . Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: “Shrunken pore syndrome”. Scand J Clin Lab Invest 2015;75:333–40. 10.3109/00365513.2015.1025427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grubb A. Shrunken pore syndrome – a common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology, and treatment options. Clin Biochem 2020;83:12–20. 10.1016/j.clinbiochem.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 45. Malmgren L, Öberg C, den Bakker Eet al. . The complexity of kidney disease and diagnosing it - cystatin C, selective glomerular hypofiltration syndromes and proteome regulation. J Intern Med 2023;293:293–308. 10.1111/joim.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Åkesson A, Lindström V, Nyman Uet al. . Shrunken pore syndrome and mortality: a cohort study of patients with measured GFR and known comorbidities. Scand J Clin Lab Invest 2020;80:412–22. 10.1080/00365513.2020.1759139. [DOI] [PubMed] [Google Scholar]
- 47. Zhang LW, Luo MQ, Xie XWet al. . Shrunken Pore Syndrome: a new and more powerful phenotype of renal dysfunction than chronic kidney disease for predicting contrast-associated acute kidney injury. J Am Heart Assoc 2023;12:e027980. 10.1161/JAHA.122.027980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khang AR, Lee MJ, Yi Det al. . The ratio of estimated glomerular filtration rate based on cystatin C and creatinine reflecting cardiovascular risk in diabetic patients. Diabetes Metab J 2023. 10.4093/dmj.2022.0177. Epub ahead of print. PMID: 36872062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roberts M, Lindheimer MD, Davison JM. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol 1996;270:F338–43. [DOI] [PubMed] [Google Scholar]
- 50. Risch L, Herklotz R, Blumberg Aet al. . Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem 2001;47:2055–9. 10.1093/clinchem/47.11.2055. [DOI] [PubMed] [Google Scholar]