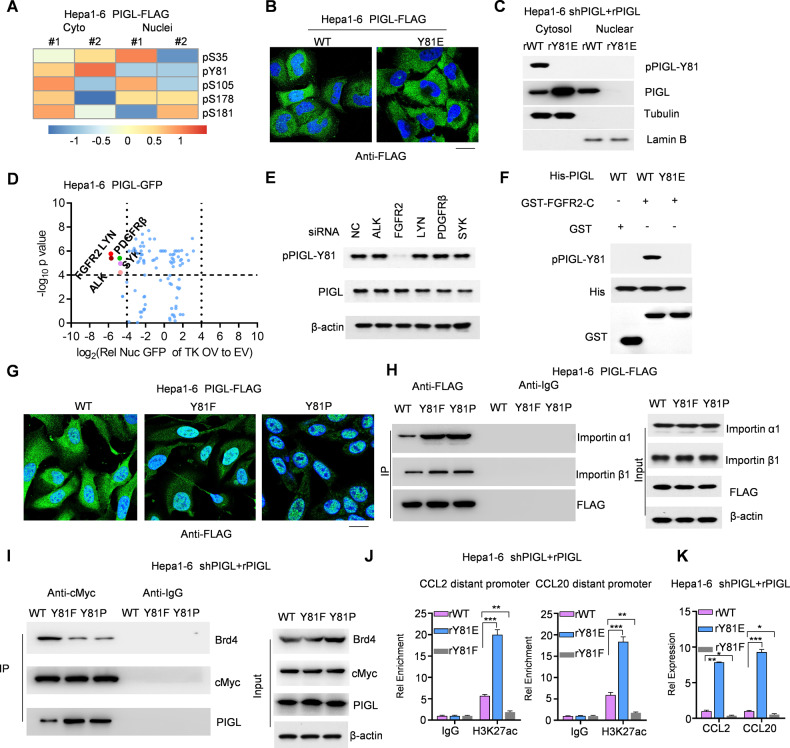
Fig. 6.
FGFR2-mediated PIGL-Y81 phosphorylation promoted PIGL retention in the cytosol and reinforced the cMyc/BRD4 axis. A PIGL-FLAG from the cytosol and nucleus was subjected to LC‒MS to identify posttranslational modifications. The detected modifications with strong intensity are summarized in the heatmap. B WT- or Y81E-PIGL-FLAG was expressed in Hepa1-6 cells and stained with an antibody against FLAG. C The cytosol and nucleus of Hepa1-6 cells expressing restored WT-PIGL or Y81E-PIGL were fractioned. D Hepa1-6 cells expressing GFP-tagged PIGL were transfected with a tyrosine kinase (TK) overexpression vector (OV), and nuclear GFP intensity was captured. The ratio of GFP intensity in the TK OV group to that in the EV group was determined and analysed. E siRNAs targeting five TK coding genes were transiently transfected into Hepa1-6 cells. F The GST-tagged intracellular C-terminus of FGFR2 was incubated with His-tagged PIGL in the kinase reaction system. G, H WT-, Y81F- or Y81P-PIGL-FLAG was stably overexpressed in Hepa1-6 cells. An antibody against FLAG was used to stain PIGL-FLAG in these cells (G), and the association of WT- and PIGL-FLAG with importin α1/β1 was ascertained using Co-IP with FLAG antibody or IgG as a blank control (H). I–K Using Hepa1-6 cells with restored expression of WT-, Y81F- or Y81P-PIGL, Co-IP with cMyc antibody or IgG as a blank control was performed to test the association of cMyc with BRD4 and PIGL (I). J, K In the indicated Hepa1-6 cells, ChIP‒qPCR using an anti-H3K27ac antibody was performed to test H3K27ac enrichment on the distant promoters of CCL2/20 (J). Readout gene expression was detected using qRT‒PCR (K). C, D, J Two-tailed Student’s t-test. (N.S., not significant; *p < 0.05; **p < 0.01; ***p < 0.001)