Abstract
Objective
To investigate sex differences in spinal radiographic progression in axial spondyloarthritis (axSpA).
Methods
AxSpA patients in the Swiss Clinical Quality Management cohort with available spinal radiographs every 2 years were included. Paired radiographs were scored by two readers according to the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS). Progression was defined as an increase of ≥2 mSASSS units in 2 years. The relationship between sex and progression was investigated with binomial generalised estimating equation models, considering baseline spinal damage as an intermediate covariate. Additional analyses included adjustments for explanatory variables and multiple imputations for missingness.
Results
In a total of 505 axSpA patients (317 men and 188 women), mean±SD radiographic progression over 2 years was 1.0±2.8 years in men and 0.3±1.1 years in women (p<0.001). Male sex was associated with enhanced progression in a small model not including baseline damage (OR 3.41, 95% CI 1.87 to 6.21). Both a direct effect of male sex on spinal progression, and an indirect effect, via enhancement of baseline spinal damage were significant (OR 2.06, 95% CI 1.15 to 3.67 and OR 1.04, 95% CI 1.01 to 1.07, respectively). A significant impact of male sex on spinal radiographic progression was still demonstrated after multiple adjustments for covariates known to potentially affect spinal radiographic progression (OR 1.97, 95% CI 1.04 to 3.71).
Conclusions
Spinal radiographic progression in axSpA is more severe in men than in women, with three times higher odds of progression in male patients and an effect that is mediated in part through an increase in baseline radiographic damage.
Keywords: spondylitis, ankylosing; outcome assessment, health care; epidemiology; tumor necrosis factor inhibitors
WHAT IS ALREADY KNOWN ON THIS TOPIC
Spinal radiographic osteoproliferative changes (syndesmophytes) in axial spondyloarthritis (axSpA) are associated with functional impairment.
Male sex has inconsistently been associated with enhanced spinal progression in analyses adjusted for known predictors of radiographic progression.
WHAT THIS STUDY ADDS
Mediation analyses demonstrate a large impact of male sex on spinal radiographic progression.
In addition to a direct effect of male sex on progression, there is an indirect effect, via an increase in baseline spinal damage, that further enhances progression in male patients.
Rare progression events in women do not allow the demonstration of retardation of progression by TNF inhibitors in the female population, contrasting findings in men.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
As inhibition of radiographic progression might be of much higher relevance in men, treatment trials should be sufficiently powered to address sex differences in axSpA.
The ensuing efforts may ultimately result in adapted international management recommendations that better consider existing sex differences in axSpA.
Introduction
Ankylosing spondylitis (AS) has traditionally been associated with male sex, particularly when the disease could only be confirmed by radiographic means.1 The advent of MRI techniques, which additionally highlighted inflammatory lesions of the axial skeleton, opened the possibility of much earlier diagnosis.2 It also provided insight into an equal sex distribution when not only earlier but also milder disease forms were included in a disease spectrum that is now called axial spondyloarthritis (axSpA).3 4 The original radiographic cut-off for sacroiliac postinflammatory damage according to the modified New York criteria is currently used to differentiate between non-radiographic and radiographic disease states (nr-axSpA and r-axSpA, respectively).5 6 It was shown to be quite arbitrary and of minor clinical relevance.7 Structural damage to the sacroiliac joints (SIJs) also has little impact on physical function.8 9 In contrast, the latter is severely impacted by osteoproliferative spinal changes (formation of syndesmophytes) and overall spinal damage.10 Disease activity, as assessed by the Ankylosing Spondylitis Disease Activity Score (ASDAS), is associated with accelerated spinal radiographic progression.11 Male sex had an independent impact on spinal progression in r-axSpA in some studies but not others.12–17 The presence of syndesmophytes was consistently found to be the most important predictor of further progression. This was even demonstrated in early disease.18 The presence of baseline damage that differs in male and female patients might conceal the true magnitude of sex differences in spinal radiographic progression if statistical analyses are adjusted for this potential intermediate variable. The aim of the study was to investigate sex differences in spinal radiographic progression over the whole axSpA disease spectrum in a large observational national registry by considering potential mediating factors and a multitude of explanatory variables.
Methods
Study population
The current analyses are nested within the ongoing Swiss Clinical Quality Management (SCQM) cohort of patients with a clinical diagnosis of axSpA, initiated in 2005.19 The period of patient inclusions and follow-ups for the current investigation stretches from 2005 to 2015. Later periods were not considered to avoid the potential influence of interleukin-17 inhibitors, which became available thereafter. The axSpA SCQM registry was initiated in 2005 and intends to assist the rheumatologist with a treat-to-target approach by providing direct feedback on disease activity measures on completion of patient and physician data entry.20 Assessments were performed according to the recommendations of the Assessment in SpondyloArthritis International Society (ASAS).21 Patient forms included the Bath Ankylosing Spondylitis Disease Activity and Functional Indices (BASDAI and BASFI, respectively), the Short Form questionnaire with 12 questions (SF-12), the European Quality of Life questionnaire with 5 domains (EQ-5D), smoking status (current, past, never), as well as a questionnaire dealing with physical activity, allowing to estimate the number of exercise sessions performed per week. Laboratory examinations included levels of C reactive protein (CRP), erythrocyte sedimentation rate and haemoglobin, as well as the human leucocyte antigen B27 (HLA-B27) status. The Swiss Rheumatology Society recommends that patients on immunosuppressive agents be examined at least annually. This is the basis for yearly clinical visits in SCQM. Additional intermediate visits are recommended at the initiation of a new treatment and 3 months after start of the respective treatment. Given the controversy related to the potential disease-modifying character of most drugs in axSpA, radiographic assessments were recommended every 2 years in SCQM (X-rays of the pelvis, of the lateral cervical and lumbar spine). Pelvis radiographs were scored centrally by two calibrated readers of the SCQM scientific committee as soon as the radiographs were submitted to SCQM to inform the treating rheumatologist about classification status (nr-axSpA vs r-axSpA).5 6
Inclusion criteria for this study were (A) fulfilment of the ASAS classification criteria for axSpA22 and (B) availability of at least two sets of lateral cervical and lumbar spine radiographs with an interval of 2 years±1 year (up to six sets of radiographs per patient over a period of 10 years).
Spinal radiographic progression
For assessment of spinal radiographic progression, cervical and lumbar radiographs were scored according to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS).23 The anterior vertebral corners (VCs) of the cervical and lumbar spinal segments (lower border of C2 to upper border of T1 and lower border of T12 to upper border of S1) are scored on lateral radiographs: erosion and/or sclerosis and/or squaring (1 point), syndesmophyte (2 points) and bridging syndesmophyte (3 points); with a total score ranging from 0 to 72. Reading was performed by two calibrated readers, rheumatologists with a long record of assessment and scoring longitudinal sets of imaging in axSpA. All the available radiographs per patients were scored at the same time, with known chronology.
Scores were not used if radiographs were missing >3 VCs per spinal segment. An adaptation algorithm24 was used if ≤3 VCs could not be scored: first, a missing value for a VC was replaced with the value of the previous observation. Second, the mean spinal segment’s progression score (either cervical or lumbar) per patient was calculated. This segmental progression score was added to the imputed value. In the case of a missing score in a patient with a score of 0 in the same VC at a subsequent time point, the score of 0 for the previous time point(s) was assumed. If the baseline score of a VC was missing, the same procedure was applied, subtracting the mean segment progression from the score of year 3 for a particular patient. If a value of this VC was also missing at year 2, then the average of the other available VCs from this spinal segment at baseline was used to replace the missing VC(s). Mean scores per VC were employed. An independent adjudicator with long experience in academic axSpA management scored all radiographs of a patient, if the mSASSS status scores between the primary readers varied ≥5 units in at least one X-ray set (N=130). In cases of adjudication, the score of the primary reader closest to the adjudicator was used. An increase in mSASSS of ≥2 units during 2 years was defined as radiographic progression according to estimates of the smallest detectable change. Radiographs with a total mSASSS≥71 were excluded, as no further progression according to this definition would be possible (N=8; all male patients). A Bland-Altman plot on 2-year progression intervals of the mSASSS to assess reliability between the two scorers for the mSASSS reading used here has already been published.25 The interobserver reliability was considered ‘good’ based on an intraclass correlation coefficient (type 2, k) of 0.85.
As an alternative definition of spinal radiographic progression, we assessed the proportion of patients with the formation of at least one new syndesmophyte over a period of 2 years. Syndesmophytes were only counted when both scorers agreed on their presence.
Statistical analysis
Baseline characteristics and clinical parameters were compared between men and women using Fisher’s exact test for nominal variables and the Mann-Whitney U test for continuous variables. A comparison of differences in means of radiographic progression in men versus women was performed using the Welch two-sample t-test.
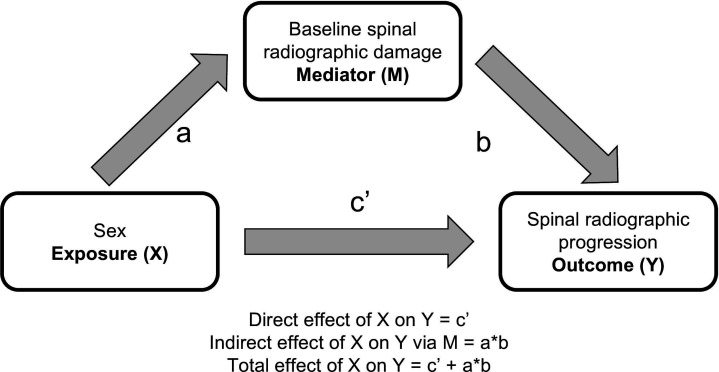
We used binomial generalised estimating equations (GEEs) to analyse the relationship between sex and radiographic progression over time. Based on previous sensitivity analyses, an ‘exchangeable’ correlation structure was chosen for all GEE models.25 The binomial family and the logistic link function were used to model the progression of ≥2 mSASSS units in 2 years. The analyses were adjusted for a multitude of parameters, chosen either as confounders or as mediators or explanatory variables according to the following principles. From a conceptual point of view, confounding seems not to be a major problem in the analyses, as it is difficult to imagine factors that affect both exposure (sex) and outcome (spinal progression). While many parameters might have a potential impact on spinal radiographic progression, they do not impact the issue of whether the individual at stake is of male or female sex. Our main statistical models were, therefore, kept very small. Given the variability of the length of the radiographic intervals allowed (2 years±12 months), we adjusted these main models for the length of the radiographic intervals. The models were also adjusted for treatment with tumour necrosis factor inhibitors (TNFi). We have chosen prior use of TNFi as the TNFi variable of choice, given its consistency in the many sensitivity analyses performed in our published work.25 Baseline spinal radiographic damage is known to be the most important predictor of further spinal progression.14 As baseline damage is also affected by sex, it was regarded as an intermediate variable for the assessment of spinal progression in the next 2 years, mediating at least part of the effect of sex on radiographic progression.14 17 As a potential mediator, baseline damage was, therefore, not included in our main statistical models to be able to capture the total effect of sex on progression (figure 1). Mediation analyses performed included the addition of baseline damage at the start of each radiographic interval to estimate the direct effect of sex on progression. The indirect effect, via baseline damage, was estimated with the Sobel test with a second-order estimator of the SE, as described by Hayes.26
Figure 1.
Potential impact of male sex on spinal progression. Given the fact that baseline spinal radiographic damage is known to be a major predictor for further radiographic progression, the diagram represents the putative effect of male sex (X) on spinal radiographic progression in the next 2 years (Y), either directly or indirectly via affecting baseline radiographic damage (baseline mSASSS or baseline presence of syndesmophytes). mSASSS, modified Stoke Ankylosing Spondylitis Spinal Score.
To allow a better prediction of radiographic progression in men and women with comparable initial presentation, we added to our main models the following time-varying or genetic covariates: symptom duration, HLA-B27 positivity, ASDAS, current smoking, number of exercise sessions per week as a proxy for mechanical strain on the spine, presence of peripheral arthritis, use of non-steroidal anti-inflammatory drugs (NSAIDs) at start of each interval (collected by the treating rheumatologist as yes/no), and body mass index (BMI) categories (either overweight (BMI 25–30 kg/m2) or obesity (BMI>30 kg/m2) compared with a reference BMI<25 kg/m2). Diagnostic delay was not considered, as it was not shown to significantly differ between the sexes in our population.27 Moreover, it demonstrated some collinearity with symptom duration. The number of exercise sessions per week was compiled from information provided by the patient on a specific questionnaire. It indicated the type of physical exercise performed (axSpA gymnastics in groups or at home, training in a fitness centre, other types of exercise) and its frequency (1–2×per week, 3–4×per week, 5–7×per week). Time-varying ASDAS-CRP was mapped to an X-ray date, if available during a timeframe of ±30 days. A time frame of 90 days before the X-ray and 30 days after the X-ray was considered for mapping the other variables to a specific X-ray date. The visit closest to the X-ray was chosen if several measurements were available during the respective timeframe.
We used multiple imputation of missing covariate data (35% of intervals had at least one missing baseline value in one of the 12 variables used in the larger model, with the proportion of missingness per variable varying from 0% to 19%). Imputation was performed separately for male and female patients. ASDAS-CRP was derived by passive imputation.
Results
Patient and disease characteristics
From 4655 patients with a clinical diagnosis of axSpA recruited to SCQM, 2977 fulfilled the ASAS classification criteria (1767 men (59.4%) and 1210 women (40.6%)). Out of these, 505 patients had at least two sets of spinal radiographs at intervals of 2 years (317 men (62.8%) and 188 women (37.2%)). The characteristics of all ASAS-positive axSpA patients (at inclusion in the SCQM cohort) and those of the subpopulation with available spinal radiographs (at the first radiograph, a time point that corresponded to the inclusion visit for most patients) are shown, stratified by sex, in table 1. Some similarities and differences between the two populations can be highlighted. While the prevalence of HLA-B27 positivity was comparable in the two populations, the percentage of patients with definite SIJ damage and the proportion of patients already treated with TNFi were higher in patients with available radiographs. Accordingly, subjective and objective disease activity parameters (BASDAI, CRP) were slightly lower in the subgroup of patients with radiographs. The impairment of spinal mobility was, however, comparable between the two populations, both for men and women. Focusing on the axSpA population with available spinal radiographs that allow the assessment of radiographic progression, men had a higher proportion of HLA-B27 positives, and were more often in the radiographic disease state and current smokers (84% vs 71%, 88% vs 75% and 39% vs 33%, for men and women, respectively). Despite a significantly higher impairment of spinal mobility in men versus women, subjective disease activity as assessed through the BASDAI, physical function (BASFI) and health-related quality of life (SF-12, EQ-5D) were comparable between the genders. A similar proportion of men and women presented with peripheral arthritis (29% vs 32%, p=0.52). The percentage of patients with an elevated CRP and the numerical value of the CRP elevation were higher in men than in women. The mean ASDAS-CRP was, accordingly, slightly higher in men in comparison to women (2.9±1.1 vs 2.7±1.0, p=0.04). The proportion of patients treated with either TNFi and/or NSAIDs was not significantly different in male versus female individuals.
Table 1.
Characteristics of axSpA patients at inclusion for all axSpA patients and at first radiograph for patients with available spinal radiographs at intervals of 2 years
| Characteristics | (A) axSpA patients fulfilling the ASAS classification criteria N=2977 |
(B) axSpA patients with available spinal radiographs N=505 |
||||||
| N 2977 |
Women N=1210 |
Men N=1767 |
P value | N 505 |
Women N=188 |
Men N=317 |
P value | |
| Age, years | 2977 | 39.3 (11.2) | 39.3 (11.6) | 0.77 | 505 | 41.5 (10.7) | 39.5 (11.2) | 0.06 |
| Symptom duration | 2910 | 11.6 (10.5) | 13.5 (11.4) | <0.001 | 497 | 13.0 (10.4) | 12.4 (9.6) | 0.67 |
| HLA-B27 positive, N (%) | 2724 | 768 (70.0) | 1368 (84.1) | <0.001 | 451 | 117 (70.9) | 240 (83.9) | 0.002 |
| r-axSpA | 1818 | 415 (61.1) | 908 (79.7) | <0.001 | 505 | 141 (75.0) | 278 (87.7) | <0.001 |
| Elevated CRP, N (%) | 2683 | 332 (30.5) | 699 (43.8) | <0.001 | 421 | 39 (26.7) | 125 (45.5) | <0.001 |
| CRP mg/L | 2698 | 8.5 (14.4) | 12.2 (17.7) | <0.001 | 422 | 7.4 (10.1) | 11.1 (12.7) | <0.001 |
| ASDAS-CRP | 2193 | 3.0 (1.0) | 2.9 (1.1) | 0.39 | 407 | 2.7 (1.0) | 2.9 (1.1) | 0.04 |
| BASDAI | 2411 | 5.1 (2.2) | 4.4 (2.3) | <0.001 | 426 | 4.5 (2.2) | 4.2 (2.3) | 0.20 |
| BASFI | 2432 | 3.4 (2.5) | 3.2 (2.6) | 0.01 | 432 | 3.0 (2.5) | 3.0 (2.5) | 0.89 |
| BASMI | 2721 | 1.6 (1.5) | 2.3 (2.1) | <0.001 | 434 | 1.6 (1.7) | 2.3 (2.5) | <0.001 |
| mSASSS | 505 | 2.3 (6.7) | 7.8 (13.5) | <0.001 | ||||
| No of syndesmophytes | 505 | 0.6 (2.3) | 2.6 (4.8) | <0.001 | ||||
| Cervical | 505 | 0.4 (1.5) | 1.2 (2.7) | <0.001 | ||||
| Lumbar | 505 | 0.2 (1.1) | 1.3 (2.9) | <0.001 | ||||
| Syndesmophytes yes/no, N (%) | 505 | 29 (15.4) | 126 (39.8) | <0.001 | ||||
| Current peripheral arthritis, N (%) | 2921 | 399 (33.6) | 495 (28.6) | 0.004 | 439 | 50 (32.0) | 82 (29.0) | 0.52 |
| EQ-5D | 2396 | 60.1 (21.2) | 63.1 (22.8) | <0.001 | 428 | 63.0 (20.7) | 65.5 (21.1) | 0.19 |
| SF-12 mental score | 2288 | 42.3 (11.4) | 44.8 (11.4) | <0.001 | 402 | 45.1 (11.3) | 46.0 (11.2) | 0.47 |
| SF-12 physical score | 2288 | 37.3 (9.9) | 39.6 (10.2) | <0.001 | 402 | 40.1 (10.1) | 40.3 (10.0) | 0.82 |
| On NSAID, N (%) | 2736 | 970 (85.9) | 1379 (85.8) | 0.96 | 400 | 122 (83.0) | 215 (85.0) | 0.67 |
| On TNF inhibitor, N (%) | 2976 | 295 (24.4) | 447 (25.3) | 0.58 | 505 | 65 (34.6) | 104 (32.8) | 0.70 |
| Current smoker, N (%) | 2381 | 303 (31.1) | 559 (39.8) | <0.001 | 426 | 50 (33.3) | 108 (39.1) | 0.25 |
| Body mass index, kg/m2 | 2776 | 24.9 (5.0) | 25.8 (4.2) | <0.001 | 430 | 24.4 (4.8) | 25.7 (3.9) | 0.002 |
| No of exercise sessions/week | 2368 | 2.5 (2.5) | 2.4 (2.5) | 0.26 | 422 | 2.3 (2.1) | 2.7 (2.7) | 0.58 |
| Radiographic intervals, N (%) | 505 | 0.50 | ||||||
| 1 interval | 216 (68.1) | 136 (72.3) | ||||||
| 2 intervals | 64 (20.2) | 36 (19.1) | ||||||
| 3 intervals | 29 (9.2) | 12 (6.4) | ||||||
| 4 intervals | 8 (2.5) | 3 (1.6) | ||||||
| 5 intervals | 0 (0.0) | 1 (0.5) | ||||||
When not otherwise mentioned, values are the mean (SD). AxSpA patients with available of ≥2 sets of spinal radiographs at intervals of 2 years (±1 year) at first radiograph. (A) All axSpA patients. (B) Patients with r-axSpA.
ASAS, Assessment in SpondyloArthritis International Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; aXSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; CRP, C reactive protein; EQ-5D, European Quality of life questionnaire with five domains; HLA-B27, human leucocyte antigen B27; mSASSS, modified Stoke Ankylosing Spondylitis Spinal Score; NSAIDs, non-steroidal anti-inflammatory drugs; r-axSpA, radiographic-aXSpA; SF-12, Short Form 12; TNF, tumour necrosis factor.
Crude spinal radiographic progression
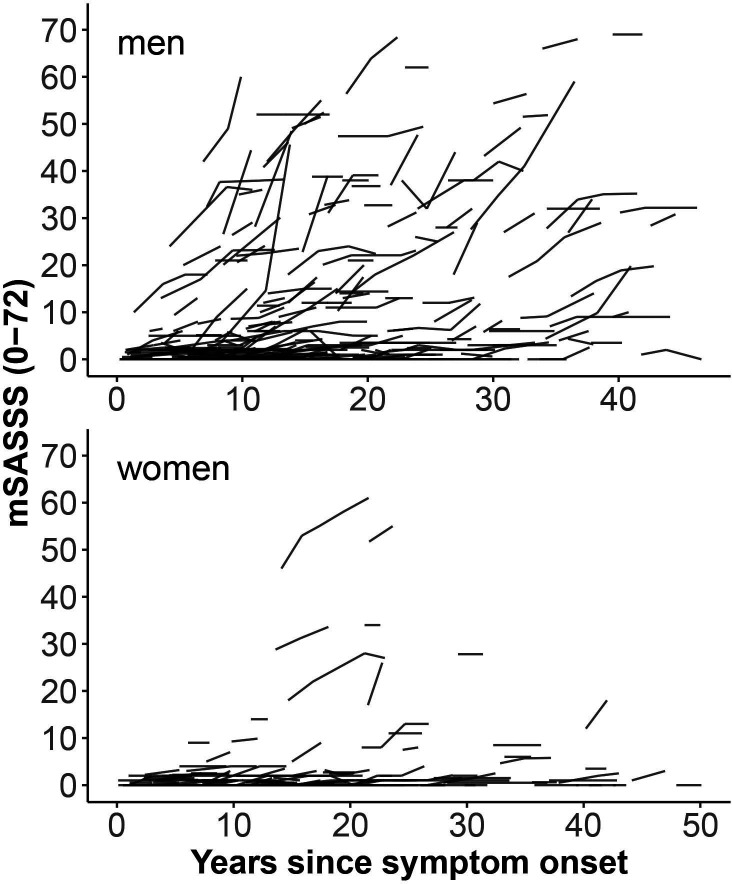
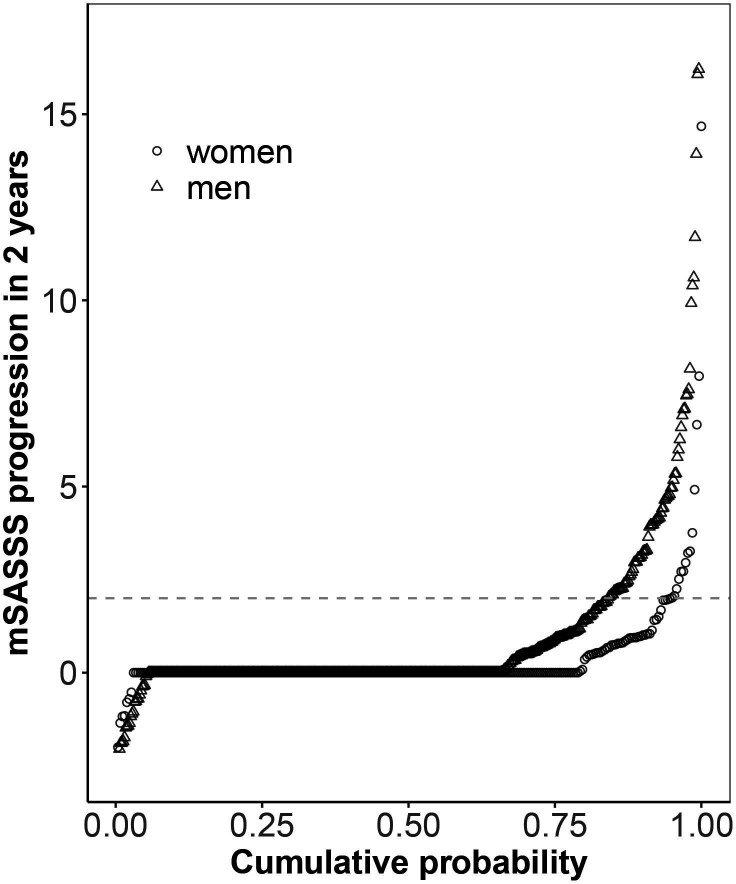
Examples of serial cervical and lumbar radiographs are shown for a woman and a man with r-axSpA in the online supplemental figures S1 and S2, respectively. In the whole population with available radiographs, a significantly higher proportion of men had syndesmophytes at baseline in comparison to women (40% vs 15%, p<0.001), with a lower mean number of syndesmophytes in women in the lumbar spine as well as in the cervical spine (table 1). In agreement with this finding, men had a significantly higher mean mSASSS (7.8 vs 2.3, p<0.001). Women had a slightly higher number of cervical than lumbar syndesmophytes, a phenomenon that was not observed in men. Patients had up to five follow-up spinal radiographs, with no differences between the sexes concerning the number of radiographic intervals (table 1). Overall mean±SD radiographic progression over 2 years in a total of 724 radiographic intervals was 0.8±2.4 with a significant difference observed between men and women (1.0±2.8 mSASSS units in men and 0.3±1.1 mSASSS units in women, p<0.001). Progression was similarly distributed over the cervical and lumbar spinal segments, both in men and women (0.46 and 0.53 mSASSS units in men and 0.18 and 0.20 mSASSS units in women, for the cervical and lumbar spine, respectively). The difference in unadjusted spinal progression observed in male and female patients is also depicted at the patient level in relation to symptom duration in figure 2 and at the level of radiographic intervals in cumulative probability plots in figure 3. These illustrations depict progression as being only present in a minority of women. Moreover, only minimal progression is observed in the first 10 years after symptom onset in female patients. The proportion of radiographic intervals affected by progression was higher in men versus women in both the cervical segment (11.9% vs 5.6%) and the lumbar segment (13.6% vs 2.2%). mSASSS progression related to symptom duration is shown stratified by sex for the cervical and the lumbar spine separately in online supplemental figure S3.
Figure 2.
Modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) for individual patients with radiographic axial spondyloarthritis plotted as a function of duration since symptom onset and stratified by sex.
Figure 3.
A 2-year progression in the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) depicted in a cumulative probability plot. The change in mSASSS values from start to end of individual 2-year radiographic intervals is shown for male patients (triangles) and for female patients (circles).
rmdopen-2023-003340supp001.pdf (1.4MB, pdf)
Adjusted analyses
In a model adjusted only for TNFi use and the length of the radiographic interval to assess the total impact of sex on spinal radiographic progression, male sex was associated with significantly higher odds of progression (OR 3.41, 95% CI 1.87 to 6.21, with progression defined as ≥2 mSASSS units in 2 years and OR 2.91, 95% CI 1.67 to 5.09, with progression defined as the appearance of at least one syndesmophyte in 2 years) (table 2). Mediation analyses of mSASSS progression as defined in figure 1 are depicted in table 3. First, we confirm an effect of sex on baseline mSASSS at the start of each X-ray interval (OR 1.83, 95% CI 1.28 to 2.61) and an effect of baseline mSASSS on spinal radiographic progression (OR 1.07, 95% CI 1.05 to 1.09). Second, we demonstrate a significant direct effect of sex on spinal progression by adding the baseline mSASSS variable to our main model (OR 2.06, 95% CI 1.15 to 3.67). The indirect effect of sex on spinal progression, via baseline mSASSS, was also significant (OR 1.04, 95% CI 1.01 to 1.07). The total impact of male sex on mSASSS progression was also analysed at the level of the cervical and lumbar spinal segments separately. The sex difference found was larger in the lumbar spine than in the cervical spine: OR 6.51, 95% CI 2.71 to 15.6 and OR 2.57, 95% CI 1.23 to 5.37, respectively (table 4). These findings were confirmed with radiographic progression defined by the appearance of at least 1 syndesmophyte in 2 years: OR 5.12, 95% CI 2.26 to 11.6 and OR 2.23, 95% CI 1.14 to 4.40 for the lumbar and the cervical spine, respectively (table 4).
Table 2.
Impact of male sex on spinal radiographic progression in axSpA
| Model | Variable | (A) Progression defined as ≥2 mSASSS units in 2 years | (B) Progression defined as ≥1 syndesmophyte in 2 years | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Main model | Male sex (total effect) | 3.41 | 1.87 to 6.21 | <0.001 | 2.91 | 1.67 to 5.09 | <0.001 |
| TNFi use prior to X-ray interval | 0.74 | 0.48 to 1.14 | 0.17 | 0.61 | 0.40 to 0.93 | 0.02 | |
| Length of the X-ray interval (+1 year) | 1.55 | 0.82 to 2.96 | 0.18 | 1.52 | 0.85 to 2.70 | 0.16 | |
| Large model | Male sex (direct effect) | 1.97 | 1.04 to 3.71 | 0.04 | 1.54 | 0.82 to 2.90 | 0.18 |
| TNFi use prior to X-ray interval | 0.60 | 0.34 to 1.05 | 0.08 | 0.58 | 0.34 to 0.97 | 0.04 | |
| Length of the X-ray interval (+1 year) | 1.78 | 0.92 to 3.45 | 0.09 | 1.99 | 1.04 to 3.78 | 0.04 | |
| mSASSS at start of X-ray interval | 1.07 | 1.05 to 1.09 | <0.001 | ||||
| Syndesmophytes at start of X-ray interval | 8.85 | 4.92 to 15.9 | <0.001 | ||||
| ASDAS (+1 unit) | 1.40 | 1.05 to 1.88 | 0.02 | 1.28 | 0.97 to 1.69 | 0.09 | |
| HLA-B27 | 0.85 | 0.41 to 1.72 | 0.64 | 0.78 | 0.38 to 1.51 | 0.43 | |
| Symptom duration (+1 year) | 1.15 | 1.01 to 1.30 | 0.03 | 1.10 | 0.98 to 1.24 | 0.12 | |
| Current smoking | 1.01 | 0.57 to 1.79 | 0.98 | 0.88 | 0.51 to 1.50 | 0.63 | |
| No of exercise sessions per week (+1 session) | 0.97 | 0.86 to 1.08 | 0.54 | 0.93 | 0.83 to 1.03 | 0.16 | |
| Current peripheral arthritis | 0.80 | 0.45 to 1.42 | 0.45 | 0.78 | 0.43 to 1.41 | 0.41 | |
| NSAIDs use at start of X-ray interval | 0.69 | 0.34 to 1.38 | 0.29 | 1.01 | 0.49 to 2.08 | 0.98 | |
| BMI 25–30 (reference BMI<25) | 1.60 | 0.93 to 2.77 | 0.09 | 1.01 | 0.61 to 1.68 | 0.97 | |
| BMI>30 (reference BMI<25) | 1.63 | 0.80 to 3.32 | 0.18 | 1.18 | 0.57 to 2.42 | 0.65 | |
Results from different multivariable models with spinal radiographic progression defined as either an increase of ≥2 mSASSS units in 2 years (A) or as the formation of ≥1 new syndesmophyte in 2 years (B), after multiple imputation of missing covariate data. Analyses performed in 724 radiographic intervals from 505 axSpA patients (108 progression events (88 in men, 20 in women) in A and 109 progression events (88 in men and 21 in women) in B.
ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BMI, body mass index; HLA-B27, human leucocyte antigen B27; mSASSS, Modified Stoke Ankylosing Spondylitis Spinal Score; NSAID, non-steroidal anti-rheumatic drug; TNFi, tumour necrosis factor inhibitor.
Table 3.
Mediation analyses of the impact of male sex on spinal radiographic progression
| Variable | OR | 95% CI | P value |
| Effect of male sex on BL mSASSS at start of each X-ray interval | 1.83 | 1.28 to 2.61 | <0.001 |
| Effect of BL mSASSS on spinal radiographic progression | 1.07 | 1.05 to 1.09 | <0.001 |
| Total effect of male sex on spinal radiographic progression | 3.41 | 1.87 to 6.21 | <0.001 |
| Direct effect of male sex on spinal radiographic progression | 2.06 | 1.15 to 3.67 | 0.01 |
| Indirect effect of male sex on spinal progression (via BL mSASSS) | 1.04 | 1.01 to 1.07 | <0.001 |
Results from different models with spinal radiographic progression defined as an increase of ≥2 mSASSS units in 2 years. The total effect of male sex on spinal progression is estimated after adjustment for prior treatment with tumour necrosis factor inhibitors and the length of the radiographic interval. The direct effect is estimated with addition of the baseline mSASSS at start of each X-ray interval to the latter model.
BL, baseline; mSASSS, modified Stoke Ankylosing Spondylitis Spinal Score.
Table 4.
Impact of male sex on spinal radiographic progression in axSpA
| Definition of progression | Variable | Cervical spine | Lumbar spine | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| ≥2 mSASSS units | Male sex (total effect) | 2.57 | 1.23 to 5.38 | 0.01 | 2.23 | 1.14 to 4.40 | 0.02 |
| TNFi use prior to X-ray interval | 0.65 | 0.39 to 1.08 | 0.10 | 0.66 | 0.40 to 1.11 | 0.12 | |
| Length of the X-ray interval (+ 1 year) | 1.04 | 0.52 to 2.07 | 0.91 | 1.16 | 0.58 to 2.32 | 0.67 | |
| ≥1 new syndesmophyte | Male sex (total effect) | 6.51 | 2.71 to 15.6 | <0.001 | 5.12 | 2.26 to 11.6 | <0.001 |
| TNFi use prior to X-ray interval | 0.98 | 0.59 to 1.62 | 0.93 | 0.54 | 0.32 to 0.91 | 0.02 | |
| Length of the X-ray interval (+ 1 year) | 1.99 | 0.96 to 4.10 | 0.06 | 0.66 | 0.77 to 3.59 | 0.20 | |
Results from different multivariable models with spinal radiographic progression defined as an increase of ≥2 mSASSS units in 2 years or as the formation of ≥1 new syndesmophyte in 2 years. Analyses performed in 724 radiographic intervals from 505 patients (59 events in the cervical spine and 64 events in the lumbar spine with progression defined as ≥2 mSASSS units and 62 events in the cervical spine and 69 events in the lumbar spine with progression defined as formation of ≥1 new syndesmophyte). The total effect of sex on radiographic progression includes a direct effect as well as in indirect effect via an impact of baseline spinal radiographic damage.
axSpA, axial spondyloarthritis; mSASSS, Modified Stoke Ankylosing Spondylitis Spinal Score; TNFi, tumour necrosis factor inhibitor.
Given the important baseline differences between men and women with regard to factors known to influence radiographic progression, we next investigated the issue, whether women displaying an initial presentation comparable to that of men would also have a comparable radiographic progression by adjusting our statistical models for a multitude of parameters impacting progression (explaining variables, not confounders). The results of these large models are shown in table 2. The amplitude of the impact of sex on spinal progression when defined as ≥2 mSASSS units in 2 years decreased but remained significant (OR 1.97; 95% CI 1.04 to 3.71, table 2A). It lost statistical significance with progression defined as the formation of at least one syndesmophyte (OR 1.54, 95% CI 0.82 to 2.90, table 2B). It is important to emphasise that baseline damage at the start of each X-ray is included and that only the direct effect of male versus female sex on progression is, therefore, estimated in these models. Complete case analyses confirmed our findings (online supplemental table S1).
The rare events of radiographic progression in women raised the question, whether retardation of radiographic progression on treatment with TNFi could be demonstrated in the female population. We set up stratified models with adjustments for a restricted number of variables and the exclusion of ASDAS, shown to be an intermediate variable. Use of TNFi prior to the radiographic interval significantly reduced the odds of progression in men but not in women (OR 0.53, 95% CI 0.31 to 0.90 and OR 1.01, 95% CI 0.31 to 3.23, for male and female patients, table 5).
Table 5.
Impact of TNFi treatment on radiographic progression in male versus female axSpA patients
| Variable | Men | Women | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| TNFi use prior to X-ray interval | 0.53 | 0.31 to 0.90 | 0.02 | 1.01 | 0.31 to 3.23 | 0.99 |
| mSASSS at start of X-ray interval | 1.05 | 1.03 to 1.08 | <0.001 | 1.14 | 1.07 to 1.20 | <0.001 |
| Length of the X-ray interval (+1 year) | 1.91 | 0.91 to 3.98 | 0.09 | 3.37 | 0.91 to 12.4 | 0.07 |
| Age (+1 year) | 1.06 | 1.02 to 1.10 | 0.001 | 1.02 | 0.96 to 1.08 | 0.54 |
| Symptom duration (+5 years) | 0.95 | 0.78 to 1.16 | 0.61 | 1.06 | 0.73 to 1.55 | 0.76 |
| No of exercise sessions/week | 0.95 | 0.85 to 1.06 | 0.34 | 0.89 | 0.66 to 1.19 | 0.42 |
Results from different multivariable models with spinal radiographic progression defined as an increase of ≥2 mSASSS units in 2 years and multiple imputation of missing covariate data. Analyses performed in 463 radiographic intervals from 317 male patients (88 events) and 261 intervals from 188 female patients (20 events) in axSpA.
axSpA, axial spondyloarthritis; mSASSS, Modified Stoke Ankylosing Spondylitis Spinal Score; TNFi, tumour necrosis factor inhibitor.
Discussion
This longitudinal study of a large observational national cohort of patients comprising the whole axSpA disease spectrum supports the notion that spinal radiographic progression is considerably greater in male compared with female patients.28
As sex is genetically determined, strict confounding is not a major issue in our main analyses, as variables may affect the outcome but not the exposure. It seems rather important to exclude potential mediators from the analyses to be able to recognise the whole amplitude of the impact of sex on progression. This seems particularly true for parameters that already differ in men and women and are known to significantly influence progression, like baseline radiographic damage and disease activity. We demonstrate both a significant direct effect of male sex on spinal progression and an indirect effect via enhancement of baseline spinal damage. The total impact of male sex on progression found here (OR 3.4) is, therefore, greater than previously demonstrated in some adjusted cohort analyses.13 15 A comparable OR of 3.98 for male sex was found in a specific multivariable analysis of the OASIS cohort in r-axSpA when the presence of baseline syndesmophytes was not incorporated.14 The important difference in radiographic progression between men and women, reflected in an OR of such magnitude, is more easily captured visually in depictions of progression in individual patients, stratified by sex, as shown here. In comparison to men, progression in women seems rather rare. The variation of progression in individual patients, with bursts of rapid progression even after long symptom duration,12 14 is predominantly observed in men. The findings are in line with the different trajectories of spinal progression identified in the PSOAS cohort, where male patients were predominantly defined as early progressors or rapid progressors, while a non-progressing or late-progressing status was more often observed in women.29
We confirm sex differences in relation to the specific spinal segments affected.12 14 Women had a slightly higher number of cervical than lumbar syndesmophytes. The cervical segment was more frequently affected by progression in women. As the opposite was observed in men, the significant difference in progression observed between male and female patients was less prominent in the cervical spine.
In clinical practice, the rheumatologist is confronted with the issue of the potential prediction of further spinal progression in an individual male or female patient with known baseline characteristics, such as the presence or absence of syndesmophytes. Does a female patient presenting with all the risk factors of further progression like a male patient really have comparable odds of progression? We dealt with this issue in the second part of our study by adjusting our models for all available parameters known to potentially impact spinal progression. As several parameters act as mediators, only a direct effect of sex on progression is identifiable in such a model, which by no means decreases the relevance of such a question. The impact of sex on spinal progression decreased after such an adjustment. While we see a trend for alignment between men and women with regard to further progression after thorough adjustment, this argument seems to miss an important point. Men and women with axSpA present in most instances differently, not only clinically and radiographically but, most importantly, also with regard to the intensity of objective signs of inflammation (as assessed by elevation of acute phase reactants or MRI).30 31 A woman presenting with a multitude of risk factors for progression comparable to male patients is seen, but infrequently, in clinical practice, and progression in female patients is, often very limited, as demonstrated here.
The spinal progression presented in our study does not represent the natural course of the disease,12 32 as our population was treated in real life and TNFi were approved for persistently active disease during the study period. Might the lower spinal progression found in women be the consequence of a better response to TNFi in women and a stronger retardation of progression on treatment with TNFi in women? Our findings here indicate the opposite, as we were not able to detect any inhibition of spinal progression in the female population, while a 50% reduction of the odds of progression was observed in men. Moreover, our previous analyses of the effectiveness of TNFi in the SCQM axSpA cohort demonstrated a significantly lower response to treatment in women in comparison to men, not only in r-axSpA but also in nr-axSpA, where the sex difference was particularly marked.33 34 Our study points to inherent differences in phenotype between the sexes. The results are in line with a recent genetic analysis of a historical Swiss cohort that revealed considerable heterogeneity in axSpA when sex and structural damage were also considered.35 Inhibition of radiographic progression might indeed be of higher relevance in men and difficult to demonstrate in women, as shown here. Indeed, a change in mSASSS on TNFi medications in groups of patients with different trajectories of progression could only be demonstrated in the rapid-progressor group, a population that mostly consisted of men.29 While there is consensus on the many unmet needs of the female population in axSpA,36 current international management recommendations37 do not overtly address potential sex issues, as randomized-controlled sex-oriented data on medications used for axSpA is scarce. Our study further emphasises the need for clinical trials in axSpA to be sufficiently powered to address sex differences.
Strengths of our analyses are the inclusion of axSpA patients representing the whole disease spectrum and the radiographic assessments every 2 years in addition to annual clinical assessments in SCQM during real-life clinical practice in academic and non-academic institutions, as well as private rheumatology practices in a relevant number of patients.38 Patients with available serial radiographs did not entirely correspond to the whole axSpA population, as they were slightly more frequently in a radiographic disease state and slightly more frequently treated with TNFi. These differences are of some benefit for an analysis of progression and its inhibition by TNFi, as spinal progression proved to be minimal in nr-axSpA.39 Only 17% of the initial population had two sets of spinal radiographs at an interval of 2 years and were included in the analysis of spinal progression. This might indicate that repeating conventional radiographs is not considered to be of any therapeutic consequence. Indeed, prevention of radiographic progression is not a dedicated aim of current management recommendations in axSpA, but is included in the respective research agenda.37 Early identification of men and women with a potential high risk of rapid progression seems a prerequisite for the demonstration of a therapeutic window of opportunity in axSpA and remains an important issue to be resolved.40
We applied multiple imputation techniques for missing values and provided complete case analyses to enable the evaluation of the robustness of our investigation.
As a limitation of our analyses, we were not able to adjust for sex differences in the amount and intensity of MRI inflammation, neither in the SIJs nor in the spine. Inflammation detected by MRI has been shown to be associated with radiographic progression and is more common in men.30 31 It would have been considered a potential mediator of the impact of sex on progression and excluded from the main analyses. Whether persisting sex differences in radiographic progression in multivariable analyses are due to failure to adjust for differences in MRI changes or other unmeasured factors remains unknown as an inherent feature of observational studies. We have used the mSASSS to assess sex differences in spinal radiographic progression.23 It remains the most validated method, but does not include the posterior aspects of the spine (particularly the facet joints).41 While a combined score has already been developed in 2017 to incorporate the assessment of the cervical facet joints in the mSASSS, it was not adopted by the international axSpA research community, as its real additional benefit remains controversial.42 The presence of degenerative osteophytes might interfere with the assessment of syndesmophytes in axSpA even in early disease,43 44 particularly in men with physically demanding jobs.45 While we cannot absolutely exclude some misinterpretation of osteoproliferative changes, trained readers are able to distinguish between these two types of lesions, and our primary readers have contributed to these studies.44 46 47
One might regard the scoring of the radiographs with known chronology as a limitation; however, it has been proven to be more sensitive to change than reading with paired time order,48 and the readers were blinded to all clinical data.
In conclusion, our data from a large cohort of patients with axSpA demonstrate a more severe spinal radiographic progression in men compared with women, a phenomenon mediated partly by an increase in baseline radiographic damage that further enhances progression in male patients.
rmdopen-2023-003340supp002.pdf (538.9KB, pdf)
rmdopen-2023-003340supp003.pdf (30.1KB, pdf)
Acknowledgments
We thank all rheumatologists and their patients for participation to SCQM. The entire SCQM staff was instrumental for data management and support. A list of rheumatology practices and hospitals that are contributing to the SCQM registries can be found on: http://www.scqm.ch/institutions.
Footnotes
Twitter: @ramicheroli
Contributors: CE, AC, RM and SK conceptualised and designed the study. AC, BM, KB, MA, MSN, OD, PE, RB and RM substantially contributed to the acquisition of clinical data. MdH and XB read the spinal radiographs with adjudication performed by AC. CE, SK, AG and AS processed the data and performed the statistical analysis. All authors contributed to interpretation of the data. CE wrote the article, and all coauthors revised the manuscript critically for important intellectual content. AC acts as guarantor and accepts full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish. All authors agreed on the final content of the submitted manuscript.
Funding: This study was funded by the Swiss Ankylosing Spondylitis Foundation, Zurich, Switzerland.
Disclaimer: The study sponsor had no role in the study design or in the collection, analysis or interpretation of the data, the writing of the manuscript or the decision to submit the manuscript for publication.
Competing interests: The SCQM foundation is supported by the Swiss Society of Rheumatology and by Abbvie, Astra Zeneca, Eli Lilly, iQone Healthcare, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Samsung Bioepis, and Sandoz. AC received honoraria for lectures from AbbVie, and Novartis. AS received consulting fees from Pfizer and support for attending meetings from Gilead. BM received speaking fees from Jansen, Novartis and Pfizer, support for attending meetings from Janssen and Pfizer, and a research grant from Celgene. MdH received grants from FWRO/FRSR and honoraria from UCB for participation in an advisory board. MJN received consulting and/or speaking fees from Abbvie, Amgen, Eli Lilly, Janssen, Novartis and Pfizer and a research grant from Novartis. He also received support from attending meetings from Janssen and UCB and participated in advisory boards for Eli Lilly, Janssen, Novartis and Pfizer. PE received financial support from UCB for attending a meeting. RM received honoraria for lectures or presentations from Abbvie, Eli Lilly, Janssen, Gilead and Pfizer. XB received consulting fees from Abbvie, BMS, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi, and UCB. He received payment or honoraria for lectures or presentations from Abbvie, BMS, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi, and UCB. He participated on advisory boards for Abbvie, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer and UCB. He is president of the Assessment of Spondyloarthritis international Society (ASAS) and EULAR president-elect. AG, CE, KB, MA, OD, RB and SK declare they have no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Restrictions apply to the availability of these data. Data are owned by a third party, the Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) foundation. Data may be obtained after approval and permission from the license holder (SCQM). Contact information for data requests: scqm@hin.ch.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethics Committee of the Canton of Zurich (KEK-ZH-Nr. 2014-0439 and BASEC 2022-00272). Participants gave informed consent to participate in the study before taking part.
References
- 1.Braun J, Sieper J. Ankylosing Spondylitis. Lancet 2007;369:1379–90. 10.1016/S0140-6736(07)60635-7 [DOI] [PubMed] [Google Scholar]
- 2.Rudwaleit M, Jurik AG, Hermann K-GA, et al. Defining active Sacroiliitis on magnetic resonance imaging (MRI) for classification of axial Spondyloarthritis: a Consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. 10.1136/ard.2009.110767 [DOI] [PubMed] [Google Scholar]
- 3.Navarro-Compán V, Sepriano A, El-Zorkany B, et al. Axial Spondyloarthritis. Ann Rheum Dis 2021;80:1511–21. 10.1136/annrheumdis-2021-221035 [DOI] [PubMed] [Google Scholar]
- 4.Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and axial Spondyloarthritis. N Engl J Med 2016;375:1303. 10.1056/NEJMc1609622 [DOI] [PubMed] [Google Scholar]
- 5.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for Ankylosing Spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 6.Boel A, Molto A, van der Heijde D, et al. Do patients with axial Spondyloarthritis with radiographic Sacroiliitis fulfil both the modified New York criteria and the ASAS axial Spondyloarthritis criteria? results from eight cohort. Ann Rheum Dis 2019;78:1545–9. 10.1136/annrheumdis-2019-215707 [DOI] [PubMed] [Google Scholar]
- 7.Ciurea A, Kissling S, Bürki K, et al. Current differentiation between radiographic and non-radiographic axial Spondyloarthritis is of limited benefit for prediction of important clinical outcomes: data from a large, prospective, observational cohort. RMD Open 2022;8:e002067. 10.1136/rmdopen-2021-002067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Protopopov M, Sieper J, Haibel H, et al. Relevance of structural damage in the Sacroiliac joints for the functional status and spinal mobility in patients with axial Spondyloarthritis: results from the German Spondyloarthritis inception cohort. Arthritis Res Ther 2017;19:240. 10.1186/s13075-017-1453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun J. Significance of structural damage in the axial skeleton in patients with axial Spondyloarthritis: how important are lesions in the Sacroiliac joint RMD Open 2022;8:e002822. 10.1136/rmdopen-2022-002822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado P, Landewé R, Braun J, et al. A stratified model for health outcomes in Ankylosing Spondylitis. Ann Rheum Dis 2011;70:1758–64. 10.1136/ard.2011.150037 [DOI] [PubMed] [Google Scholar]
- 11.Ramiro S, van der Heijde D, van Tubergen A, et al. Higher disease activity leads to more structural damage in the spine in Ankylosing Spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. 10.1136/annrheumdis-2014-205178 [DOI] [PubMed] [Google Scholar]
- 12.Baraliakos X, Listing J, von der Recke A, et al. The natural course of radiographic progression in Ankylosing Spondylitis – evidence for major individual variations in a large proportion of patients. J Rheumatol 2009;36:997–1002. 10.3899/jrheum.080871 [DOI] [PubMed] [Google Scholar]
- 13.Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated acute-phase Reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial Spondyloarthritis. Arthritis Rheum 2012;64:1388–98. 10.1002/art.33465 [DOI] [PubMed] [Google Scholar]
- 14.van Tubergen A, Ramiro S, van der Heijde D, et al. Development of new Syndesmophytes and bridges in Ankylosing Spondylitis and their predictors: a longitudinal study. Ann Rheum Dis 2012;71:518–23. 10.1136/annrheumdis-2011-200411 [DOI] [PubMed] [Google Scholar]
- 15.Deminger A, Klingberg E, Geijer M, et al. A five-year prospective study of spinal radiographic progression and its predictors in men and women with Ankylosing Spondylitis. Arthritis Res Ther 2018;20:162. 10.1186/s13075-018-1665-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Slik B, Spoorenberg A, Wink F, et al. Although female patients with Ankylosing Spondylitis score worse on disease activity than male patients and improvement in disease activity is comparable, male patients show more radiographic progression during treatment with TNF-Α inhibitors. Semin Arthritis Rheum 2019;48:828–33. 10.1016/j.semarthrit.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 17.Koo BS, Oh JS, Park SY, et al. Tumour necrosis factor inhibitors slow radiographic progression in patients with Ankylosing Spondylitis: 18-year real-world evidence. Ann Rheum Dis 2020;79:1327–32. 10.1136/annrheumdis-2019-216741 [DOI] [PubMed] [Google Scholar]
- 18.Ramiro S, Heijde D, Sepriano A, et al. Spinal radiographic progression in early axial Spondyloarthritis: five-year results from the DESIR cohort. Arthritis Care Res 2019;71:1678–84. 10.1002/acr.23796 Available: https://onlinelibrary.wiley.com/toc/21514658/71/12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciurea A, Scherer A, Exer P, et al. Tumor necrosis factor Α inhibition in radiographic and Nonradiographic axial Spondyloarthritis: results from a large observational cohort. Arthritis Rheum 2013;65:3096–106. 10.1002/art.38140 [DOI] [PubMed] [Google Scholar]
- 20.Smolen JS, Braun J, Dougados M, et al. Treating Spondyloarthritis, including Ankylosing Spondylitis and Psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis 2014;73:6–16. 10.1136/annrheumdis-2013-203419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieper J, Rudwaleit M, Baraliakos X, et al. The assessment of Spondyloarthritis International society (ASAS) Handbook: a guide to assess Spondyloarthritis. Ann Rheum Dis 2009;68 Suppl 2:ii1–44. 10.1136/ard.2008.104018 [DOI] [PubMed] [Google Scholar]
- 22.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of Spondyloarthritis International society classification criteria for axial Spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 23.Creemers MCW, Franssen MJAM, van’t Hof MA, et al. Assessment of outcome in Ankylosing Spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. 10.1136/ard.2004.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in Ankylosing Spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis 2015;74:52–9. 10.1136/annrheumdis-2013-204055 [DOI] [PubMed] [Google Scholar]
- 25.Molnar C, Scherer A, Baraliakos X, et al. TNF blocker inhibit spinal radiographic progression in Ankylosing Spondylitis by reducing disease activity: results form the Swiss clinical quality management cohort. Ann Rheum Dis 2018;77:63–9. 10.1136/annrheumdis-2017-211544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford Press, 2013. [Google Scholar]
- 27.Ciurea A, Scherer A, Weber U, et al. Age at symptom onset in Ankylosing Spondylitis: is there a gender difference Ann Rheum Dis 2014;73:1908–10. 10.1136/annrheumdis-2014-205613 [DOI] [PubMed] [Google Scholar]
- 28.Wright GC, Kaine J, Deodhar A. Understanding differences between men and women with axial Spondyloarthritis. Semin Arthritis Rheum 2020;50:687–94. 10.1016/j.semarthrit.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 29.Hwang MC, Lee M, Gensler LS, et al. Identifying Trajectories of radiographic spinal disease in Ankylosing Spondylitis: a 15-year follow-up study of the PSOAS cohort. Rheumatology (Oxford) 2022;61:2079–87. 10.1093/rheumatology/keab661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro-Compán V, Ramiro S, Landewé R, et al. Disease activity is Longitudinally related to Sacroiliac inflammation on MRI in male patients with axial Spondyloarthritis: 2-years of the DESIR cohort. Ann Rheum Dis 2016;75:874–8. 10.1136/annrheumdis-2015-207786 [DOI] [PubMed] [Google Scholar]
- 31.Baraliakos X, Listing J, Rudwaleit M, et al. The relationship between inflammation and new bone formation in patients with Ankylosing Spondylitis. Arthritis Res Ther 2008;10:R104. 10.1186/ar2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brody S, Mackay K, Al-Saidi A, et al. The natural history of Ankylosing Spondylitis as defined by radiological progression. J Rheumatol 2002;29:1236–43. [PubMed] [Google Scholar]
- 33.Hebeisen M, Neuenschwander R, Scherer A, et al. Response to tumor necrosis factor inhibition in male and female patients with Ankylosing Spondylitis: data from a Swiss cohort. J Rheumatol 2018;45:506–12. 10.3899/jrheum.170166 [DOI] [PubMed] [Google Scholar]
- 34.Neuenschwander R, Hebeisen M, Micheroli R, et al. Differences between men and women with Nonradiographic axial Spondyloarthritis: clinical characteristics and treatment effectiveness in a real-life prospective cohort. Arthritis Res Ther 2020;22:233. 10.1186/s13075-020-02337-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, van der Linden SM, Khan MA, et al. Heterogeneity of axial Spondyloarthritis: Genetics, sex and structural damage matter. RMD Open 2022;8:e002302. 10.1136/rmdopen-2022-002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chimenti M-S, Alten R, D’Agostino M-A, et al. Sex-associated and gender-associated differences in the diagnosis and management of axial Spondyloarthritis: addressing the unmet needs of female patients. RMD Open 2021;7:e001681. 10.1136/rmdopen-2021-001681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial Spondyloarthritis: 2022 update. Ann Rheum Dis 2023;82:19–34. 10.1136/ard-2022-223296 [DOI] [PubMed] [Google Scholar]
- 38.Ciurea A, Weber U, Stekhoven D, et al. Treatment with tumor necrosis factor inhibitors in axial Spondyloarthritis: a comparison between private rheumatology practices and academic centers in a large observational cohort. J Rheumatol 2015;42:101–5. 10.3899/jrheum.140229 [DOI] [PubMed] [Google Scholar]
- 39.Hebeisen M, Micheroli R, Scherer A, et al. Spinal radiographic progression in axial Spondyloarthritis and the impact of classification as Nonradiographic versus radiographic disease: data from the Swiss clinical quality management cohort. PLoS One 2020;15:e0230268. 10.1371/journal.pone.0230268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson PC, Brown MA. The window of opportunity: a relevant concept for axial Spondyloarthritis. Arthritis Res Ther 2014;16:109. 10.1186/ar4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Heijde D, Braun J, Deodhar A, et al. Modified Stoke Ankylosing Spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in Ankylosing Spondylitis. Rheumatology (Oxford) 2019;58:388–400. 10.1093/rheumatology/key128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maas F, Arends S, Brouwer E, et al. Incorporating assessment of the Cervical facet joints in the modified Stoke Ankylosing Spondylitis spine score is of additional value in the evaluation of spinal radiographic outcome in Ankylosing Spondylitis. Arthritis Res Ther 2017;19:77. 10.1186/s13075-017-1285-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Bruin F, ter Horst S, Bloem HL, et al. Prevalence of degenerative changes of the spine on magnetic resonance images and Radiographs in patients aged 16-45 years with chronic back pain of short duration in the Spondyloarthritis caught early (SPACE) cohort. Rheumatology (Oxford) 2016;55:56–65. 10.1093/rheumatology/kev283 [DOI] [PubMed] [Google Scholar]
- 44.de Bruin F, Treyvaud MO, Feydy A, et al. Prevalence of degenerative changes and overlap with Spondyloarthritis-associated lesions in the spine of patients from the DESIR cohort. RMD Open 2018;4:e000657. 10.1136/rmdopen-2018-000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramiro S, Landewé R, van Tubergen A, et al. Lifestyle factors may modify the effect of disease activity on radiographic progression in patients with Ankylosing Spondylitis: a longitudinal analysis. RMD Open 2015;1:e000153. 10.1136/rmdopen-2015-000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baraliakos X, Listing J, Rudwaleit M, et al. Progression of radiographic damage in patients with Ankylosing Spondylitis: defining the central role of Syndesmophytes. Ann Rheum Dis 2007;66:910–5. 10.1136/ard.2006.066415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baraliakos X, Listing J, Buschmann J, et al. A comparison of new bone formation in patients with Ankylosing Spondylitis and patients with diffuse idiopathic Skeletal Hyperostosis: a retrospective cohort study over six years. Arthritis Rheum 2012;64:1127–33. 10.1002/art.33447 [DOI] [PubMed] [Google Scholar]
- 48.Wanders AJB, Landewé RBM, Spoorenberg A, et al. What is the most appropriate Radiologic scoring method for Ankylosing Spondylitis? A comparison of the available methods based on the outcome measures in rheumatology clinical trials filter. Arthritis Rheum 2004;50:2622–32. 10.1002/art.20446 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003340supp001.pdf (1.4MB, pdf)
rmdopen-2023-003340supp002.pdf (538.9KB, pdf)
rmdopen-2023-003340supp003.pdf (30.1KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Restrictions apply to the availability of these data. Data are owned by a third party, the Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) foundation. Data may be obtained after approval and permission from the license holder (SCQM). Contact information for data requests: scqm@hin.ch.