Background:
Neoadjuvant therapy remains controversial in treating resectable pancreatic ductal adenocarcinoma (PDAC) patients. This study aims to assess the impact of neoadjuvant therapy on survival in patients with PDAC according to their clinical stage.
Methods:
Patients with resected clinical Stage I–III PDAC from 2010 to 2019 were identified in the surveillance, epidemiology, and end results database. A propensity score matching method was utilized within each stage to reduce potential selection bias between patients who underwent neoadjuvant chemotherapy followed by surgery and patients who underwent upfront surgery. An overall survival (OS) analysis was performed using the Kaplan–Meier method and a multivariate Cox proportional hazards model.
Results:
A total of 13 674 patients were included in the study. The majority of the patients (N=10 715, 78.4%) underwent upfront surgery. Patients receiving neoadjuvant therapy followed by surgery had significantly longer OS than those with upfront surgery. Subgroup analysis revealed that the neoadjuvant chemoradiotherapy group’s OS is comparable to neoadjuvant chemotherapy. In clinical Stage IA PDAC, there was no difference in survival between the neoadjuvant treatment and upfront surgery groups before or after matching. In stage IB-III patients, neoadjuvant therapy followed by surgery improved OS before and after matching compared to upfront surgery. The results revealed the same OS benefits using the multivariate Cox proportional hazards model.
Conclusion:
Neoadjuvant therapy followed by surgery could improve OS over upfront surgery in Stage IB-III PDAC but did not provide a significant survival advantage in Stage IA PDAC.
Keywords: neoadjuvant therapy, pancreatic cancer, SEER database, upfront surgery
Introduction
Highlights
The benefits of neoadjuvant therapy for pancreatic ductal adenocarcinoma (PDAC) vary depending on the stage.
Patients with stage IB-III PDAC benefited from neoadjuvant therapy before surgery.
Neoadjuvant treatment does not improve survival in stage IA PDAC patients.
More accurate staging is needed when neoadjuvant therapy is used.
Pancreatic cancer is one of the leading reasons of cancer death worldwide, with an estimated 62 210 new cases and 49 830 deaths in the United States in 20211. The most pathological type of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC)2. Over 80% of patients are diagnosed with advanced pancreatic cancer at diagnosis, which significantly reduces the likelihood of curative resection. In addition, the 5-year survival rate for patients with resectable lesions that undergo surgical resection is only 15–25%3. The role of adjuvant chemotherapy has been considered standard management for PDAC after surgical resection and has been shown to improve overall survival (OS) significantly4,5.
Decisions about the resectability status of PDAC should be made in consensus at multidisciplinary discussions, which usually depends on radiological criteria for the contact between tumor and peripheral blood vessels. Resectable PDAC is defined as no arterial or venous tumor contact or tumor contact with the superior mesenteric vein or portal vein less than or equal to 180°. Solid tumor contact with the superior mesenteric artery less than 180°, or with arteries or venous invasion, which allows for safe and complete resection and reconstruction belongs to borderline resectable PDAC. Locally advanced disease is defined as solid tumor contact greater than 180° with the arteries or unreconstructible venous invasion6.
Neoadjuvant therapy is becoming increasingly prevalent in treating several malignant tumors, such as nonsmall-cell lung cancer and triple-negative and HER2-positive breast cancer7,8. Regarding PDAC, neoadjuvant therapy followed by surgery leads to higher rates of R0 and N0 resection rates than surgery performed immediately after diagnosis9. Neoadjuvant therapy could significantly improve the OS in patients with borderline resectable and locally advanced PDAC. Nevertheless, the optimal treatment sequence for resectable PDAC remains controversial since not all clinical trials indicate a statistically significant difference in OS10–13. As recently reported by Shoucair et al. 14, matrix metalloproteinase seven expression in fine-needle aspiration biopsies is associated with pathologic response to neoadjuvant therapy in resected PDAC patients. As a result of this study, we understood the different benefits of neoadjuvant therapy of PDAC from the viewpoint of molecular biology. This result also reminded us that more specific guidance might be obtained with more detailed stratification of resectable patients.
This study aimed to perform a propensity-matched analysis (PSM) comparing neoadjuvant therapy followed by resection with upfront surgery in patients with resectable and borderline resectable PDAC, with a stage-specific analysis for stage I–III PDAC patients.
Methods
Study population and study design
This study used a retrospective cohort collected from the Surveillance, Epidemiology, and End Results (SEER) database, a program that records cancer diagnoses, treatment, and survival data for the population of the United States. Our analysis has been approved by SEER Research Plus Data (November 2021 submission) (Username:14376-Nov2018). To include patients who have undergone a more standardized neoadjuvant treatment, only PDAC patients with positive histopathology between 2010 and 2019 have been included in the study. Additional information included basic patient information, the American Joint Committee on Cancer (AJCC) TNM stage, tumor size, tumor site, treatment options, surgical type, and follow-up information. Furthermore, the eighth edition clinical stage was calculated based on the tumor size and positive lymph nodes’ presence. We excluded the following patients from this study: those whose survival time was less than one month, those with distant metastases, patients who did not undergo surgery or with an unclear surgical type, those whose T or N stage was unclear, and those undergoing only neoadjuvant radiotherapy (Fig. 1). The term neoadjuvant therapy refers to the administration of chemoradiotherapy or chemotherapy before surgery. Following subgroup stratification, these patients were analyzed according to Stages IA, IB, IIA, IIB, III-N2, and III-T4. The study was complied with the 1964 Helsinki Declaration, and the work had been reported in line with the strengthening the reporting of cohort, cross-sectional and case-control studies in surgery (STROCSS) Criteria15, Supplemental Digital Content 1, http://links.lww.com/JS9/A431. This study was exempted by the ethics committee of the Tianjin Medical University Cancer Institute and Hospital because the SEER database is publicly available, and all patient data have been unlabeled.
Figure 1.
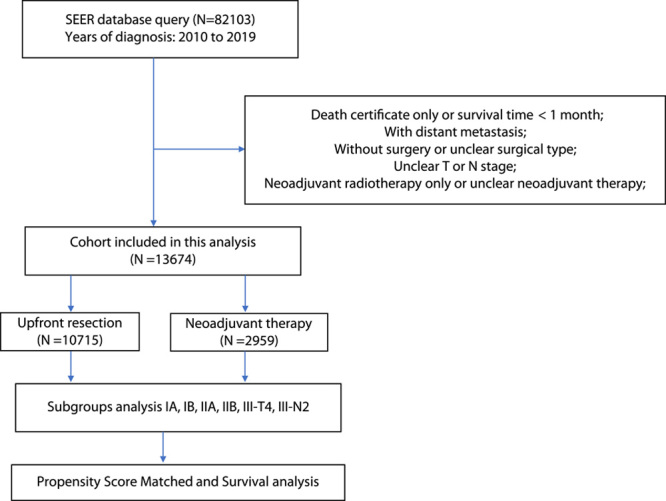
Procedures for inclusion and exclusion of the cohort.
Statistical Analyses
The continuous variables were compared using the student t-test and expressed as the mean and SD. Categorical variables were summarized as numbers and percentages and compared using χ 2-tests or Fisher’s exact tests. A PSM (1:1 or 2:1, without replacement) was conducted to eliminate the influence of baseline variables in each subgroup. This study’s primary objective was to determine OS. OS is defined as the time from the diagnosis to the death. We evaluated the significance of the difference in survival among the upfront surgery and neoadjuvant therapy groups using Kaplan–Meier survival curves with the Log-Rank test and multivariable Cox proportional hazard regression analyses. The statistical analyses were conducted using R version 4.2.1 (http://www.r-project.org/). P-values less than 0.05 in a two-tailed test were considered statistically significant.
Results
Demographic characteristics and survival of the whole cohort and resectable patients
The cohort of this study consisted of 13 674 patients with stage I–III PDAC. A total of 10 715 patients underwent upfront surgery, and 2959 underwent neoadjuvant therapy. There were significant differences in baseline characteristics factors among upfront surgery and neoadjuvant therapy groups (Table 1). The mean age of patients in the upfront surgery group was older than the neoadjuvant therapy group (67.6 years vs. 64.4 years, P<0.001). The ratio of tumors in the head of the pancreas was significantly higher in the neoadjuvant therapy group compared to the upfront surgery group, and a higher ratio of patients in the neoadjuvant therapy group underwent pancreatoduodenectomy (P<0.001). T4 tumor accounted for 16.3% of the neoadjuvant therapy group, which was significantly higher than the upfront surgery group (3.1%) (P<0.001). Regarding adjuvant therapy, a higher proportion of patients undergoing upfront surgery (70.5%) and radiotherapy (23.6%) receive it than those receiving neoadjuvant therapy.
Table 1.
Baseline variables, intraoperative information, and adjuvant treatment between neoadjuvant therapy followed by surgery and upfront surgery Groups in the whole original cohort
| Neoadjuvant Therapy (N=2959) % | Upfront Surgery (N=10 715) % | P | |
|---|---|---|---|
| Marital status | <0.001 | ||
| Married | 2012 (68.0) | 6641 (62.0) | |
| Unmarried | 947 (32.0) | 4074 (38.0) | |
| Race | 0.021 | ||
| White | 2467 (83.4) | 8749 (81.7) | |
| Black | 262 (8.9) | 958 (8.9) | |
| Other | 230 (7.8) | 1008 (9.4) | |
| Age | <0.001 | ||
| Mean (SD) | 64.4 (9.54) | 67.6 (10.2) | |
| Gender | 0.755 | ||
| Female | 1447 (48.9) | 5275 (49.2) | |
| Male | 1512 (51.1) | 5440 (50.8) | |
| Site | <0.001 | ||
| Head | 2255 (76.2) | 7685 (71.7) | |
| Body/Tail | 429 (14.5) | 2140 (20.0) | |
| Other/Overlapping | 275 (9.3) | 890 (8.3) | |
| Surgery | <0.001 | ||
| Pancreatoduodenectomy | 2312 (78.1) | 7858 (73.3) | |
| Partial pancreatectomy | 315 (10.6) | 1648 (15.4) | |
| Total pancreatectomy | 332 (11.2) | 1209 (11.3) | |
| Examined nodal number | <0.001 | ||
| <10 | 539 (18.2) | 2089 (19.5) | |
| 11–19 | 1133 (38.3) | 4464 (41.7) | |
| 20+ | 1287 (43.5) | 4162 (38.8) | |
| Adjuvant chemotherapy | <0.001 | ||
| No | 1767 (59.7) | 3159 (29.5) | |
| Yes | 1192 (40.3) | 7556 (70.5) | |
| Adjuvant radiotherapy | <0.001 | ||
| No | 2524 (85.3) | 8184 (76.4) | |
| Yes | 435 (14.7) | 2531 (23.6) | |
| T | <0.001 | ||
| T1 | 275 (9.3) | 1733 (16.2) | |
| T2 | 1652 (55.8) | 6218 (58.0) | |
| T3 | 551 (18.6) | 2445 (22.8) | |
| T4 | 481 (16.3) | 319 (3.0) | |
| N | <0.001 | ||
| N0 | 1515 (51.2) | 3548 (33.1) | |
| N1 | 973 (32.9) | 4202 (39.2) | |
| N2 | 471 (15.9) | 2965 (27.7) | |
| Stage | <0.001 | ||
| IA | 148 (5.0) | 962 (9.0) | |
| IB | 826 (27.9) | 1831 (17.1) | |
| IIA | 260 (8.8) | 660 (6.2) | |
| IIB | 825 (27.9) | 4084 (38.1) | |
| III | 900 (30.4) | 3178 (29.7) |
The median survival was higher in the neoadjuvant therapy group compared with the upfront surgery group (29 months vs. 22 months, P<0.001, Fig. 2A). In addition, we removed patients with the T4 stage to get cohorts with resectable disease. The Kaplan–Meier survival curves revealed that the median survival of the neoadjuvant therapy group was also significantly higher than the upfront surgery group in patients with resectable disease (29 months vs. 22 months, P<0.001, Fig. 2B). Then we compared the survival impact of neoadjuvant chemotherapy with neoadjuvant chemoradiotherapy. The results show that the survival impact of neoadjuvant chemotherapy is comparable to that of neoadjuvant chemoradiotherapy in the whole neoadjuvant cohort (29 months vs. 29 months, P=0.678, Fig. 2C) and resectable cohort (29 months vs. 28 months, P=0.244, Fig. 2D).
Figure 2.
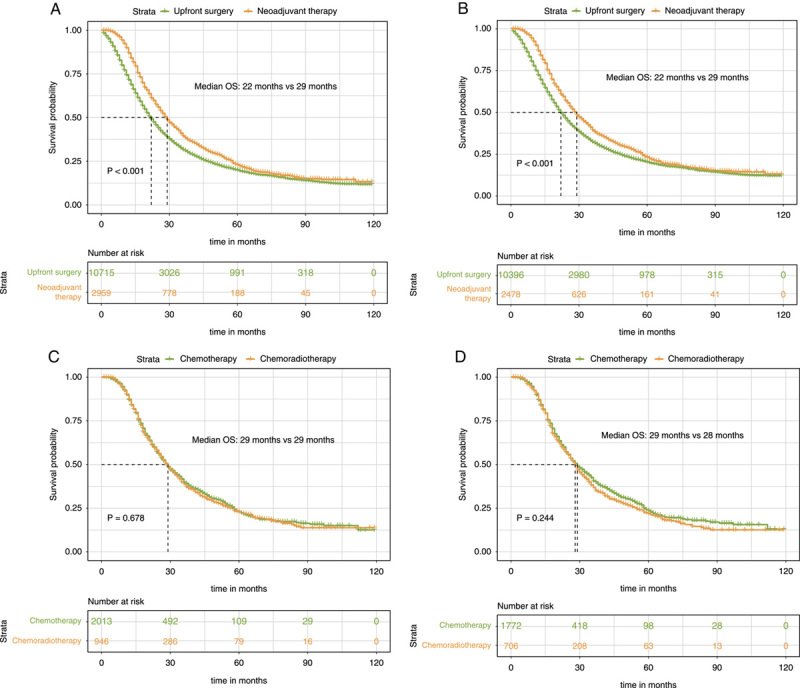
Kaplan–Meier survival curves for overall survival between patients of the upfront resection group and patients of the neoadjuvant therapy followed by surgery group for the whole cohort (A) and resectable cohort (B). Kaplan–Meier survival curves for overall survival between patients of the neoadjuvant chemotherapy group and patients of the neoadjuvant chemoradiotherapy group for the whole cohort (C) and resectable cohort (D).
Subgroup analysis of the survival outcomes
We conducted PSM in the stage-specific analysis of stage I–III patients to balance the distribution of baseline variables (Supplement Table 1s–6 , Supplemental Digital Content 2, http://links.lww.com/JS9/A432). After PSM, there was no significant difference between upfront surgery and neoadjuvant therapy groups at baseline characteristics of each stage of patients. To further investigate the survival outcome between the patients who underwent upfront surgery and neoadjuvant therapy in different disease stages, the Kaplan–Meier survival analysis was performed in each subgroup both before and after PSM.
Of the patients with Stage IA PDAC, the OS was similar between upfront surgery and the neoadjuvant therapy group either in both unmatched (53 months vs. 35 months, P=0.396, Fig. 3A) or matched data (49 months vs. 35 months, P=0.306, Fig. 4A). For Stage IB patients, median OS was statistically significantly improved in the neoadjuvant therapy group compared with the upfront surgery subgroup before (32 months vs. 29 months, P=0.001, Fig. 3B) and after PSM (32 months vs. 30 months, P=0.05, Fig. 4B). Additionally, neoadjuvant therapy also significantly correlated with a longer survival time in Stage IIA patients for both unmatched (34 months vs. 22 months, P<0.001, Fig. 3C in the Supplement) and matched patients (36 months vs. 24 months, P<0.001, Fig. 4C). Likewise, for patients with stage IIB, patients who underwent neoadjuvant therapy had significantly longer OS than patients who receive upfront surgery before (26 months vs. 21 months, P<0.001, Fig. 3D) and after PSM (26 months vs. 23 months, P=0.004, Fig. 4D).
Figure 3.
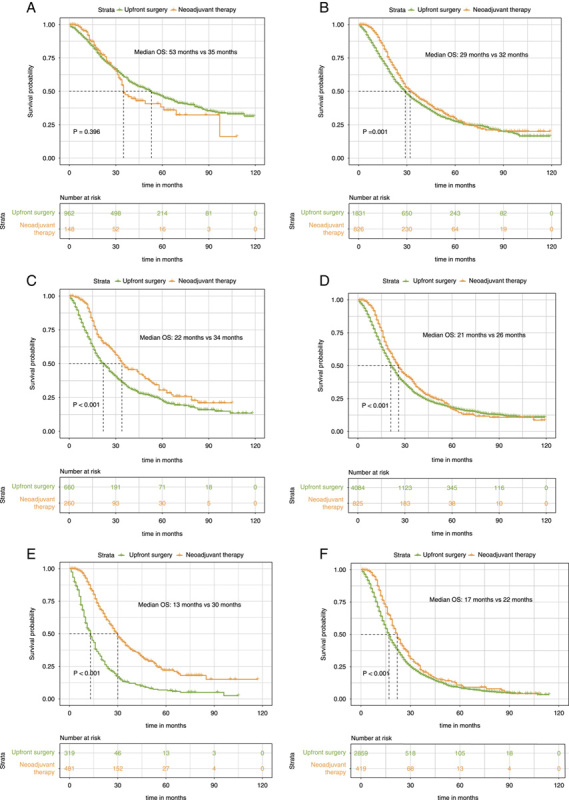
Kaplan–Meier survival curves for overall survival between patients of the upfront resection group and patients of the neoadjuvant therapy followed by surgery group for clinical stage IA (A), clinical stage IB (B), and clinical stage IIA (C), clinical stage IIB (D), clinical stage III-T4 (E), and clinical stage III-N2 (F) patients in the unmatched data.
Figure 4.
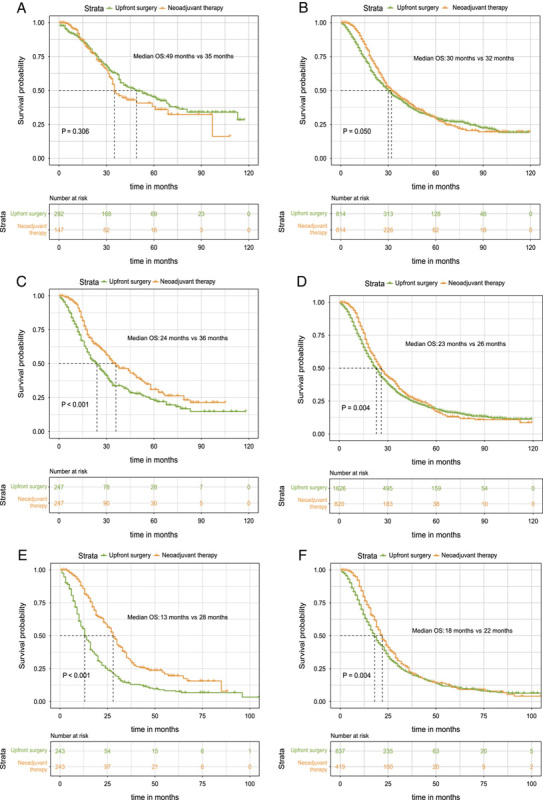
Kaplan–Meier survival curves for overall survival between patients of the upfront resection group and patients of the neoadjuvant therapy followed by surgery group for clinical stage IA (A), clinical stage IB (B), and clinical stage IIA (C), clinical stage IIB (D), clinical stage III-T4 (E), and clinical stage III-N2 (F) patients in the matched data.
The patients with stage III-T4 belong to the borderline resectable category. Notably, we also found that OS was significantly improved for the neoadjuvant therapy group for both unmatched (30 months vs. 13 months, P<0.001, Fig. 3E) and matched patients (28 months vs. 13 months, P<0.001, Fig. 4E) compared with those receiving upfront surgery. The double median survival benefit also highlighted the important role of neoadjuvant therapy in borderline resectable PDAC. Finally, in patients with stage III-N2 disease, significant improvement in median OS was also discovered before (22 months vs. 17 months, P<0.001, Fig. 3F) and after PSM (22 months vs. 18 months, P=0.04, Fig. 4F). Therefore, except for patients with the IA stage, patients in other specific stages could receive survival benefits after neoadjuvant treatment.
Neoadjuvant therapy compared to upfront surgery plus adjuvant chemotherapy
The NCCN guidelines recommend adjuvant chemotherapy for patients with pancreatic cancer who have had initial surgery because it can significantly improve survival. Though it is challenging in the real world for every patient who underwent upfront surgery to receive adjuvant chemotherapy, we conduct a further subgroup analysis to compare the survival impact of neoadjuvant therapy with upfront surgery plus adjuvant chemotherapy in order to improve the credibility of the benefits of neoadjuvant therapy. As Figures 5A, B, D, and F showed, the OS was comparable between upfront surgery plus adjuvant chemotherapy and neoadjuvant therapy group in patients with stage IA (53 months vs. 35 months, P=0.086), stage IB (36 months vs. 32 months, P=0.287), stage IIB (26 months vs. 26 months, P=0.917), and stage IIIB (21 months vs. 22 months, P=0.556). However, the neoadjuvant therapy group could still achieve significantly longer OS than the upfront surgery plus adjuvant chemotherapy group in patients with stage IIA (36 months vs. 26 months, P=0.010), III-T4 (28 months vs. 16 months, P<0.001). This result highlights the significance of neoadjuvant therapy in the management of Stage IIA and III-T4 disease.
Figure 5.
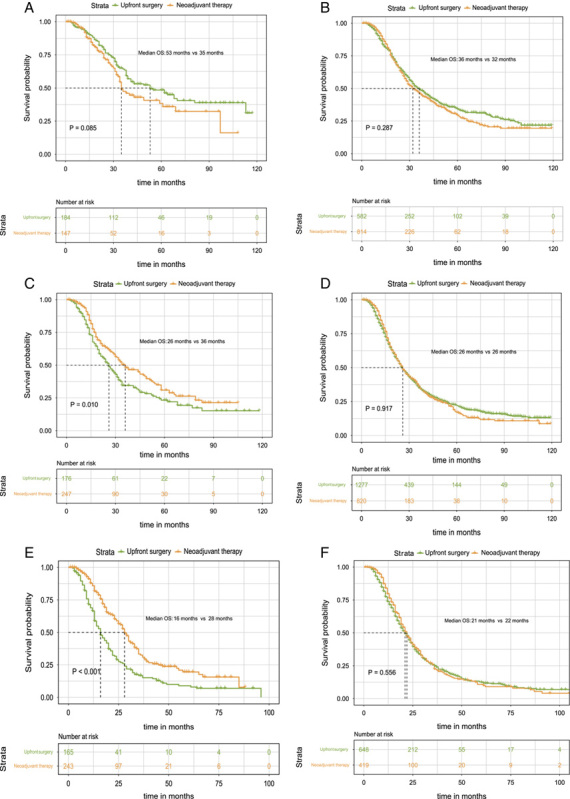
Kaplan–Meier survival curves for overall survival between patients of the upfront resection plus adjuvant chemotherapy group and patients of the neoadjuvant therapy followed by surgery group for clinical Stage IA (A), clinical stage IB (B), and clinical stage IIA (C), clinical stage IIB (D), clinical stage III-T4 (E), and clinical stage III-N2 (F) patients in the matched data.
Subgroup multivariate Cox analysis of the role of neoadjuvant therapy
To further reduce the bias of baseline information and adjuvant chemotherapy on neoadjuvant effects, subgroup multivariable Cox proportional hazards regression analyses were conducted to further evaluate the hazard ratio (HR) and 95% CI of OS of neoadjuvant therapy in different subgroups of specific stages in matched patients. Except in patients with Stage IA HR=1.216; 95% CI, 0.874–1.692; P=0.246; Fig. 6A), neoadjuvant therapy was demonstrated as an independent prognosis factor which could significantly improve OS in patients with stage IB (HR=0.737; 95% CI, 0.633–0.859; P<0.001; Fig. 6B), IIA (HR=0.646; 95% CI, 0.497–0.840; P=0.001; Fig. 6C), IIB (HR=0.660; 95% CI, 0.583–0.748; P<0.001; Fig. 7A), III-T4 (HR=0.394; 95% CI, 0.310–0.500; P<0.001; Fig. 7B), and III-N2 (HR=0.631; 95% CI, 0.537–0.742; P<0.001; Fig. 7C). The OS benefits were independent of age, sex, tumor location, and adjuvant therapy. Thus, these results further confirmed the role of neoadjuvant treatment in improving OS in stage I–III PDAC instead of patients with stage IA.
Figure 6.
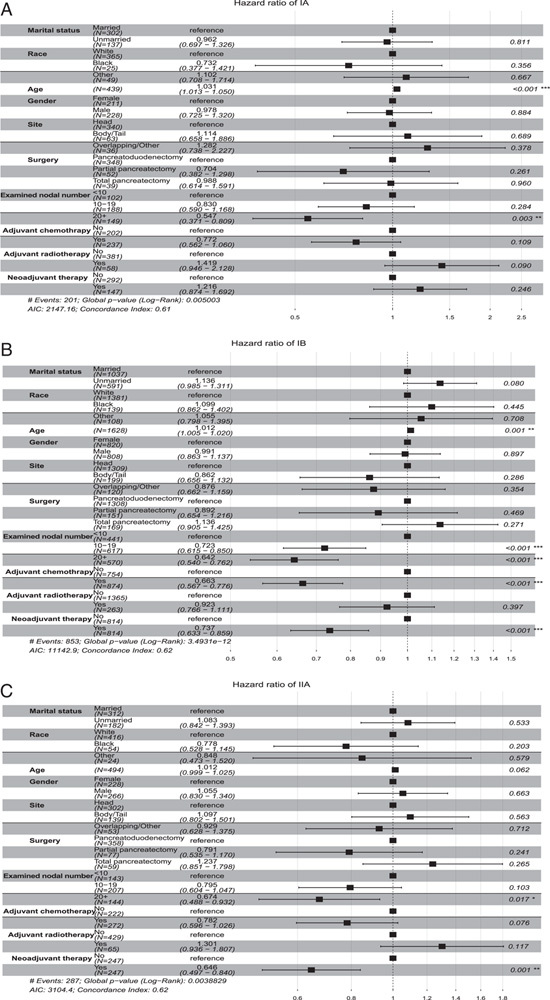
Multivariate Cox regression analysis with Hazard ratios represented for overall survival in matched data of clinical stage IA (A), clinical stage IB (B), and clinical stage IIA (C) patients.
Figure 7.
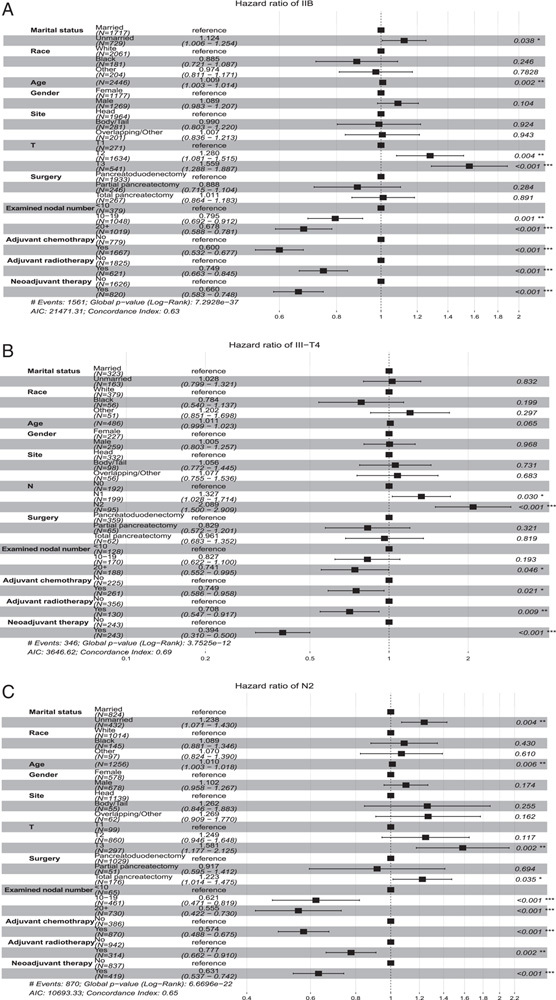
Multivariate Cox regression analysis with Hazard ratios represented for overall survival in matched data of clinical stage IIB (A), clinical stage III-T4 (B), and clinical stage III-N2 (C) patients.
Discussion
It is essential to conduct multimodality therapy for the curative treatment of PDAC. To the best of our knowledge, this study is the first to assess neoadjuvant therapy’s role in subgroups of PDAC stratified by the eighth TNM stage. The result of our study found that patients with stage IB-III PDAC who received neoadjuvant therapy followed by surgery had a better OS than those treated conventionally upfront surgery. It is noteworthy that for patients with stage IA PDAC, the OS was comparable for those who received neoadjuvant therapy followed by surgery and those who underwent surgery upfront.
This study aimed to determine whether neoadjuvant therapy improved the survival rate in nonmetastasized PDAC at stage-specific stages. Evidence has increasingly refuted that neoadjuvant therapy is essential for borderline resectable PDAC, as it may enhance the R0 resection rate and prolong the patient’s survival time11,16,17. In line with the above results, our data also demonstrated that patients undergoing adjuvant therapy followed by surgery had a more prolonged OS than those undergoing upfront surgery for borderline resectable PDAC (stage III-T4). Additionally, a tremendous improvement in median survival time was observed in stage III-T4 patients (17 months in the unmatched cohort and 15 months in the matched cohort). However, this study could only analyze the T4 tumors, which is the anatomical definition of borderline resectable pancreatic cancer based on anatomic criteria alone. The recent international consensus has defined patients with borderline resectable pancreatic cancer according to three distinct dimensions: anatomical, biological, and conditional18. They are all associated with prognosis, which in turn may help to decide treatment strategy19. It is also very interesting to explore the role of neoadjuvant therapy in the conditional and biological definitions of borderline resectable pancreatic cancer in future studies.
However, neoadjuvant treatment of resectable PDAC remains controversial20,21. We explored this further by stratifying the subgroup analyses based on the eighth TNM stage. Neoadjuvant therapy followed by surgery improved survival over stage IB-III patients who underwent upfront surgery. However, we discovered that neoadjuvant therapy followed by surgery did not significantly improve OS in patients with very early-stage PDAC (Stage IA) compared to the upfront surgery. Furthermore, the median OS of the neoadjuvant therapy group followed by surgery was even lower than that of the upfront surgery group (35 months vs. 53 months in the matched and 35 months vs. 49 months in the unmatched cohorts, respectively). For the intention-to-treat analysis, it is important to note that our cohort excluded those patients who lost an opportunity to undergo surgery due to tumor progression during neoadjuvant therapy, which might significantly impact survival in the neoadjuvant therapy group. Further multivariate Cox analysis of our study also revealed that stage IA is the only subgroup that could not benefit from neoadjuvant therapy independently. According to the above analysis, neoadjuvant treatment may prove ineffective for patients with stage IA pancreatic cancer. As a result, we do not recommend undergoing neoadjuvant treatment for patients with stage IA pancreatic cancer before applying a novel neoadjuvant strategy with better therapeutic responses. As a result of these different therapeutic efficacy differences, the molecular biology and immune microenvironment of stage IA tumors may differ from those of other stages, which has been proven to be correlated with the efficacy of neoadjuvant therapy in other malignant tumors22–24. This hypothesis is worthy of further exploration in future research.
Another interesting finding in our study was that the OS of patients who received neoadjuvant chemotherapy and chemoradiotherapy was comparable in this cohort. Several clinical trials have recently been conducted to determine the optimal strategy of neoadjuvant therapy for PDAC. Patients with PDAC who received total neoadjuvant therapy (chemotherapy plus radiation) achieved a higher rate of complete pathologic response than those who received only neoadjuvant chemotherapy25. However, one study found that despite a higher rate of negative margin resection with total neoadjuvant therapy, it was associated with a more unfavorable 90-day mortality than classical neoadjuvant chemotherapy and failed to demonstrate a survival benefit over classical neoadjuvant chemotherapy alone26. Additionally, a recent randomized controlled trial found that neoadjuvant mFOLFIRINOX treatment alone was associated with a favorable OS in patients with borderline resectable PDAC compared to mFOLFIRINOX treatment combined with hypofractionated radiation27. It is, therefore, worth investigating whether neoadjuvant chemotherapy alone or total neoadjuvant therapy is the most effective therapeutic schedule.
It is important to note that our study has several limitations. First, this is a retrospective cohort study that unmeasured factors could confound. Second, we could not conduct an intention-to-treat analysis in this study because patients who received neoadjuvant therapy intending to undergo surgery and did not undergo surgery could not be identified in the SEER database. As a third critical bias, the specific chemotherapy and radiotherapy data for neoadjuvant therapy are unavailable in the SEER database, which leaves the treatment details regimens unclear. To ensure that most patients in this study had received more standardized neoadjuvant therapy, we included only patients diagnosed after 2010. Furthermore, our study did not evaluate the pathological response to neoadjuvant treatment due to the lack of information in SEER databases. In addition, due to the relevant information being unavailable from the SEER database, we could not compare the quality of life and postoperative morbidity between the two groups. Finally, we could not analyze two significant surgical results, R0 and N0, due to insufficient permissions in the SEER database.
Conclusion
Neoadjuvant therapy followed by surgery would benefit all patients with PDAC except those at stage IA. In the case of resectable PDAC, a more precise staging would be beneficial in making a clinical decision regarding the use of neoadjuvant therapy.
Ethical approval
This is a SEER database analysis article with no ethical requirements.
Sources of funding
This work was supported by the National Key Research and Development Program of China (2021YFA1201100), the National Natural Science Foundation of China (grants 82030092, 82072657, 82072716, 82072659, 82272680, 82173295, 82072691, 82272767, 82273362, 82271895, 82103006, 82103222, 82273259, 82273284, 82272799, 82203402, 82203019 and 82203169), Tianjin Natural Science Fundation (19JCJQJC63100), Tianjin Research Innovation Project for Postgraduate Students (2021YJSB262 and 2022BKY162), Tianjin Key Medical Discipline (Specialty) Construction Projec(TJYXZDXK-009A), Collaboration Program of Beijing Tianjin and Hebei (22JCZXJC00120).
Author contribution
Y.P.Z., J.H.H., J.Y., S.G., and C.G.: conceived and designed the experiments; Y.P.Z., X.Y., T.X.Z., Y.J.X., X.F.G., and J.Y.: acquisition, analysis and interpretation of data; Y.P.Z. and J.Y.: drafted the manuscript; J.H.H.: obtained funding; J.H.H., J.Y., and C.G.: study supervision. All authors revised the manuscript and agreed with the manuscript’s results and conclusions.
Conflicts of interest disclosure
The authors declare no competing interests.
Research Registration Unique Identifying Number (UIN)
1. Name of the registry: Research Resitry.
2. Unique Identifying number or registration ID: researchregistry8689.
3. Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/browse-theregistry#home/registrationdetails/63f198d0e82c41002726389b/.
Data availability statement
The data that support the findings of this study are openly available in SEER database.
Guarantor
Jun Yu, MD, PhD, Department of Medicine, Johns Hopkins University, 5501 Hopkins Bayview Circle, Baltimore, MD 21224, USA. E-mail: jyu41@jhmi.edu
Supplementary Material
Footnotes
J.Y. and J.H.H. are co-corresponding authors.
Y.Z., S.G. and X.Y. contributed equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.journal-surgery.net.
Published online 3 May 2023
Contributor Information
Yiping Zou, Email: yiping-zou@foxmail.com.
Song Gao, Email: foxgao2004@163.com.
Xin Yu, Email: yuxin202301@163.com.
Tianxing Zhou, Email: zhoutianxing1995@qq.com.
Yongjie Xie, Email: 2335940013@qq.com.
Xiaofan Guo, Email: anyecloudy@163.com.
Ran An, Email: anran201608@163.com.
Xiuchao Wang, Email: wangxiuchao2008@163.com.
Tiansuo Zhao, Email: zhaotiansuo@tjmuch.com.
Antao Chang, Email: changantao@tjmuch.com.
Chuntao Gao, Email: gaochuntao@tjmuch.com.
Jun Yu, Email: jyu41@jhmi.edu.
Jihui Hao, Email: haojihui@tjmuch.com.
References
- 1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 2. Wood LD, Hruban RH. Pathology and molecular genetics of pancreatic neoplasms. Cancer J 2012;18:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol 2021;18:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267–277. [DOI] [PubMed] [Google Scholar]
- 5. Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073–1081. [DOI] [PubMed] [Google Scholar]
- 6. Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J National Comp Cancer Net 2021;19:439–57. [DOI] [PubMed] [Google Scholar]
- 7. Leon-Ferre RA, Hieken TJ, Boughey JC. The landmark series: neoadjuvant chemotherapy for triple-negative and HER2-positive breast cancer. Ann Surg Oncol 2021;28:2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saw SPL, Ong BH, Chua KLM, et al. Revisiting neoadjuvant therapy in non-small-cell lung cancer. Lancet Oncol 2021;22:e501–e16. [DOI] [PubMed] [Google Scholar]
- 9. van Dam JL, Janssen QP, Besselink MG, et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer 2022;160:140–149. [DOI] [PubMed] [Google Scholar]
- 10. Golcher H, Brunner TB, Witzigmann H, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol 2015;191:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the dutch randomized PREOPANC trial. J Clin Oncol 2022;40:1220–30. [DOI] [PubMed] [Google Scholar]
- 12. Reni M, Balzano G, Zanon S, et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol 2018;3:413–23. [DOI] [PubMed] [Google Scholar]
- 13. Rangelova E, Wefer A, Persson S, et al. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg 2021;273:579–86. [DOI] [PubMed] [Google Scholar]
- 14. Shoucair S, Chen J, Martinson JR, et al. Association of matrix metalloproteinase 7 expression with pathologic response after neoadjuvant treatment in patients with resected pancreatic ductal adenocarcinoma. JAMA Surg 2022;157:e221362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mathew G, Agha R. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 16. Jang JY, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg 2018;268:215–22. [DOI] [PubMed] [Google Scholar]
- 17. Yamaguchi J, Yokoyama Y, Fujii T, et al. Results of a Phase II Study on the use of neoadjuvant chemotherapy (FOLFIRINOX or GEM/nab-PTX) for borderline-resectable pancreatic cancer (NUPAT-01). Ann Surg 2022;275:1043–1049. [DOI] [PubMed] [Google Scholar]
- 18. Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2–11. [DOI] [PubMed] [Google Scholar]
- 19. Hayasaki A, Isaji S, Kishiwada M, et al. Survival analysis in patients with pancreatic ductal adenocarcinoma undergoing chemoradiotherapy followed by surgery according to the international consensus on the 2017 definition of borderline resectable cancer. Cancers 2018;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Geus SW, Eskander MF, Bliss LA, et al. Neoadjuvant therapy versus upfront surgery for resected pancreatic adenocarcinoma: a nationwide propensity score matched analysis. Surgery 2017;161:592–601. [DOI] [PubMed] [Google Scholar]
- 21. Lof S, Korrel M, van Hilst J, et al. Impact of neoadjuvant therapy in resected pancreatic ductal adenocarcinoma of the pancreatic body or tail on surgical and oncological outcome: a propensity-score matched multicenter study. Ann Surg Oncol 2020;27:1986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yam C, Yen EY, Chang JT, et al. Immune phenotype and response to neoadjuvant therapy in triple-negative breast cancer. Clin Cancer Res 2021;27:5365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Yu M, Jing Y, et al. Baseline immunity and impact of chemotherapy on immune microenvironment in cervical cancer. Br J Cancer 2021;124:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Z, Gao X, Peng X, et al. Multi-omics characterization of molecular features of gastric cancer correlated with response to neoadjuvant chemotherapy. Sci Adv 2020;6 eaay4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barrak D, Villano AM, Villafane-Ferriol N, et al. Total neoadjuvant therapy for pancreatic adenocarcinoma increases probability for a complete pathologic response. Eur J Surg Oncol 2022;48:1356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oba A, Wu YHA, Colborn KL, et al. Comparing neoadjuvant chemotherapy with or without radiation therapy for pancreatic ductal adenocarcinoma: national cancer database cohort analysis. The. Br J Surg 2022;109:450–454. [DOI] [PubMed] [Google Scholar]
- 27. Katz MHG, Shi Q, Meyers J, et al. Efficacy of preoperative mfolfirinox vs mfolfirinox plus hypofractionated radiotherapy for borderline resectable adenocarcinoma of the pancreas: the A021501 phase 2 randomized clinical trial. JAMA Oncol 2022;8:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in SEER database.


