Abstract
The bacterial divisome is a macromolecular machine composed of more than 30 proteins that controls cell wall constriction during division. Here, we present a model of the structure and dynamics of the core complex of the E. coli divisome, supported by a combination of structure prediction, molecular dynamics simulation, single-molecule imaging, and mutagenesis. We focus on the septal cell wall synthase complex formed by FtsW and FtsI, and its regulators FtsQ, FtsL, FtsB, and FtsN. The results indicate extensive interactions in four regions in the periplasmic domains of the complex. FtsQ, FtsL, and FtsB support FtsI in an extended conformation, with the FtsI transpeptidase domain lifted away from the membrane through interactions among the C-terminal domains. FtsN binds between FtsI and FtsL in a region rich in residues with superfission (activating) and dominant negative (inhibitory) mutations. Mutagenesis experiments and simulations suggest that the essential domain of FtsN links FtsI and FtsL together, potentially modulating interactions between the anchor-loop of FtsI and the putative catalytic cavity of FtsW, thus suggesting a mechanism of how FtsN activates the cell wall synthesis activities of FtsW and FtsI.
Subject terms: Cellular microbiology, Single-molecule biophysics, Bacterial structural biology, Cell division
The divisome is a macromolecular machine composed of more than 30 proteins that controls cell wall constriction during bacterial cell division. Here, the authors provide insights into the structure and dynamics of the divisome core complex using a combination of structure prediction, molecular dynamics simulation, single-molecule imaging, and mutagenesis.
Introduction
Bacterial cell division is an essential process and a frequent target for novel antibiotics1–3. The cytoskeletal protein FtsZ defines the future division site4 and recruits more than thirty proteins, many of them cell-wall enzymes and regulators. Collectively termed the divisome5,6, these proteins orchestrate cell-wall constriction during cell division. In Escherichia coli, the essential septal peptidoglycan (sPG) polymerase FtsW and its cognate transpeptidase FtsI form the sPG synthase complex FtsWI and cooperate to synthesize new septal cell wall7,8. FtsWI activities are regulated by a conserved subcomplex of transmembrane proteins FtsQ, FtsL, and FtsB (FtsQLB)9–12, and by FtsN in γ-proteobacteria13. However, how the septal cell wall synthesis activities of FtsWI are regulated by FtsQLB and FtsN remains unclear.
In E. coli, FtsN has been regarded as the trigger of septum synthesis, as its arrival at the division site coincides with the initiation of cell-wall constriction13. FtsN and FtsN-like proteins are conserved in γ-proteobacteria and contain SPOR domains that bind denuded glycans14. In E. coli, an essential, periplasmic segment of FtsN (FtsNE) is sufficient to initiate constriction in the absence of full-length FtsN13,15,16. Our recent single-molecule studies show that FtsNE is part of a processive sPG synthesis complex with active FtsWI and required to maintain the processivity of the FtsWI complex17,18. Whether FtsQLB is also part of this complex and, if so, how it associates with FtsWI, is unknown. In vitro experiments using purified proteins found that E. coli FtsQLB inhibits FtsI activity19, while Pseudomonas aeruginosa FtsQLB enhances FtsW activity9. In neither case did the addition of FtsN impact FtsI or FtsW activity. In vivo experiments showed that mutations in FtsL, FtsW, and FtsI can be either dominant negative (DN) or superfission (SF, bypassing FtsN and/or complementing DN mutations)9,10,15,20,21. These seemingly contradictory observations suggest that FtsWI can transition between on and off states depending on the environment and/or genetic mutations, and that FtsQLB and FtsN may play important roles in shifting FtsWI between the two states10,11,20. However, it is unclear what conformations of FtsWI correspond to the on and off states and how FtsN shifts FtsWI between the two states.
A recent review synthesized evidence to date and used structure predictions to propose a model of allosteric regulation of FtsWI in which multiple interactions cooperatively stabilize an active conformation22. This work drew on structure predictions of complexes FtsWI, FtsQLB, and FtsLWI using AlphaFold2 (AF2)23. Structure prediction was also recently applied to other divisome interactions20,24. While AF2 makes predictions that are largely consistent with available structural data and can accurately predict protein–protein interfaces25–27, its predictions can fail to distinguish between different states of the same complex28 or describe the consequences of point mutations29.
In this work, we provided experimental evidence to show that FtsQLB forms a complex with active FtsWI in vivo and conducted structure predictions for E. coli FtsWI, FtsWI in complex with FtsQLB (FtsQLBWI), and for FtsQLBWI in complex with FtsNE (FtsQLBWIN). We subjected these predicted structures to all-atom molecular dynamics (MD) simulation to observe dynamics of protein–protein interfaces on the microsecond timescale. We observed extensive interactions in the transmembrane and periplasmic domains of the FtsQLBWI and FtsQLBWIN complexes. Next, we carried out mutagenesis and single-molecule tracking experiments to investigate these observed interactions. Further MD simulations using DN and SF point mutations revealed critical conformational changes at FtsQLB-FtsI protein–protein interfaces and near the putative catalytic region of FtsW. Collectively, our results support a model in which FtsQLB scaffolds FtsWI in an extended conformation poised for activation, with FtsNE functioning as a tether between FtsI and FtsL to impact the interactions between the anchor-loop of FtsI and the catalytic cavity of FtsW, suggesting an activation mechanism of FtsWI by FtsN.
Results
Single-molecule tracking suggests that FtsQLB remains in complex with FtsWI on both FtsZ and sPG tracks
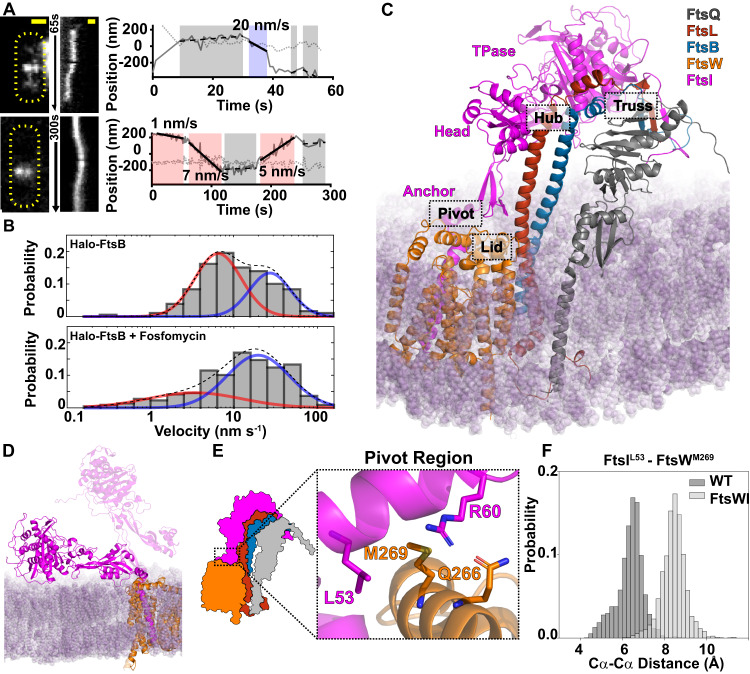
Previously, using single-molecule tracking, we showed that FtsWI exhibits two directionally moving subpopulations that reflect their activities in sPG synthesis:17,30 a fast-moving subpopulation (~30 nm/s) driven by FtsZ treadmilling dynamics30,31 and inactive in sPG synthesis (on the Z track) and a slow-moving subpopulation (~8 nm/s) independent of FtsZ treadmilling and driven by active sPG synthesis (on the sPG track). We later observed that processive FtsN exhibits only a slow-moving population, indicating association with FtsWI in an sPG synthesis complex on the sPG track18. To investigate whether FtsQLB is part of the processive, active complex on the sPG track and/or part of the inactive complex on the Z track, we ectopically expressed a Halo-FtsB fusion protein that complemented an FtsB depletion strain (Fig. S1A, Tables S1 and S2). We sparsely labeled Halo-FtsB with a fluorescent dye, JF646, and performed single-molecule tracking as we previously described17.
We observed that a large percentage of single Halo-FtsB molecules exhibited directional motion (53.7 ± 2.7%, μ ± s.e.m., n = 379 segments) (Fig. 1B, top, Movie S1, and Movie S2). The velocity distribution of directional segments of Halo-FtsB single-molecule trajectories was best described as the sum of fast- and slow-moving subpopulations (vfast = 32.9 ± 5.2 nm/s, vslow = 8.2 ± 1.7 nm/s, pfast = 38% ± 13%, μ ± s.e.m., n = 179 segments, Fig. 1B, bottom, S1B, and Table S3). In the presence of fosfomycin, a drug that inhibits the synthesis of FtsW substrate Lipid II, Halo-FtsB shifted to the fast-moving subpopulation (vfast = 28.5 ± 2.9 nm/s, vslow = 8.5 ± 3.0 nm/s, pfast = 70% ± 10%, μ ± s.e.m., n = 218 segments, Figs. 1B, S1C, and Table S3). The existence of two subpopulations of FtsB and the response to fosfomycin (Figs. 1B, S1B, S1C, and Table S3) mirrored observations for FtsW in our previous study17, but differed from those of FtsN, which exhibited only slow motion18. Since FtsQ, FtsL, and FtsB form a stable heterotrimer32, these observations suggest that FtsQLB is in complex with both fast-moving, inactive FtsWI on the Z track17 and slow-moving, active FtsWI on the sPG track, with the latter also including FtsN18. As such, FtsWI in complex with FtsQLB may be able to adopt both active and inactive states, which are regulated by FtsN.
Fig. 1. Characterization and modeling of the E. coli FtsQLBWI complex.
A, B Single-molecule tracking of Halo-FtsB suggests that FtsQLB remain in complex with FtsWI on both the fast-moving FtsZ-track and the slow-moving sPG synthesis track. A Two representative Halo-FtsB expressing cells with the maximum fluorescence intensity projection images (left), kymographs of fluorescence line scans at the midcell (middle), and unwrapped one-dimensional positions of the corresponding Halo-FtsB molecule along the circumference (solid gray line) and long axis (dotted gray line) of the cell were shown. Measured velocity of each segment and the corresponding classification (fast-moving, cyan; slow-moving, pink; stationary, gray) are labeled in the trajectory panels. Scale bar 500 nm. Similar images were observed in N > 100 cells. B Distribution of velocities of single Halo-FtsB molecules exhibiting directional motion in wild-type E. coli cells grown in minimal media in the absence (top) of fosfomycin was best fit with two moving populations, one slow (red) and one fast (blue). A dashed line indicates the summed probability. In the presence of fosfomycin that inhibits cell wall synthesis (bottom), the slow-moving population (red) is drastically reduced. These dynamics behaviors are similar to those of FtsW or FtsI17. C Modeled structure of E. coli FtsQLBWI within a POPE bilayer (purple) in the last frame of a 1-μs MD simulation. The complex consists of FtsQ 20–276 (gray), FtsL 1–121 (red), FtsB 1–113 (blue), FtsW 46–414 (orange), and FtsI 19–588 (magenta). The FtsI TPase, head and anchor domains are labeled in magenta text. The four interface regions—Pivot, Truss, Hub, and Lid—are highlighted in dashed boxes. D In the absence of FtsQLB, FtsI (magenta) collapses to the membrane (purple) at the end of the 1-μs MD simulation. The position of FtsI at the beginning of the simulation (transparent magenta) is shown for comparison. E Zoomed-in view of the Pivot region in FtsQLBWI, in which interactions between FtsIL53 (magenta) and FtsWM269 (orange) and between FtsIR60 (magenta) and FtsWQ266 (orange) secure the position of the FtsI anchor domain (magenta) with respect to FtsW (orange). F In the absence of FtsQLB, interactions between FtsIL53 and FtsWM269 are broken, as shown by the increased Cα-Cα distances between the two residues (light gray) compared to that in the presence of FtsQLB (WT, dark gray) in the last 500 ns of the MD simulation. Source data are provided as a Source Data file.
An FtsQLBWI model describes an extended conformation of FtsI and extensive protein–protein interfaces
To gain insight into how the conformation of FtsWI can adopt both active and inactive states for sPG synthesis in the FtsQLBWI complex, we used the ColabFold33 implementation of AlphaFold2 (AF2)23 to predict the atomic structure of FtsQLBWI. In the prediction, we did not utilize template coordinates, avoiding explicit dependence on homologous published structures. The predicted structure of the complex showed an extended conformation of FtsI supported by interactions between the membrane-distal C-terminal domains of FtsQ, FtsL, FtsB, and FtsI, as well as interactions between transmembrane helices (TMH) of FtsL, FtsB, FtsW, and FtsI. These interactions were predicted with high local confidence (pLDDT) except for extreme terminal residues that have no predicted interactions (Fig. S2A). Furthermore, predicted DockQ values34 for protein–protein interfaces with individual subunits ranged from 0.64 to 0.73 (Table S4), indicating accurate predictions of protein complexes27. Finally, predictions for homologous complexes of FtsQLBWI in other diderm (gram negative) and monoderm (gram positive) species were largely similar, with notable differences at less conserved C-terminal domains, such as the different interactions reported for PASTA domains in FtsI homologs Bacillus subtilis Pbp2B35 and Streptococcus pneumonia Pbp2X36 (Fig. S2B). These results suggest that AF2 can accurately predict protein–protein interfaces of E. coli FtsQLBWI and homologous complexes, but it remains essential to verify that predictions are consistent with experimental data and predictive in new experiments.
Next, we built a system of FtsQLBWI in a lipid bilayer (simulation details in Table S5) and performed a 1 µs all-atom molecular dynamics (MD) simulation to investigate the stability of predicted protein–protein interfaces and conformational dynamics of the complex. We excluded cytoplasmic, N-terminal regions of FtsQ (residues 1–19), FtsW (1–45), and FtsI (1–18) on the basis of having very low pLDDT and/or lacking high-confidence predicted protein–protein interactions. The MD simulation revealed a dynamic complex, with the FtsI transpeptidase (TPase) domain tilting backward and the head domain wrapping around the FtsL helix (Figs. 1C, S3 and Movie S3). Unless otherwise noted, we describe and depict structures after 1 µs of MD hereafter. Throughout the complex, we observed extensive interactions reported in previous experimental studies, such as leucine-zipper-like interactions between FtsL and FtsB helices37 (Fig. S4A), and interactions between the same FtsW and FtsI transmembrane helices observed for the paralog RodA-PBP238,39 (Fig. S4B). The resulting complex is also consistent with crystal structures of E. coli FtsI (Fig. S5A)40 and a partial FtsQ-FtsB complex (Fig. S5B)41. Finally, our model of FtsQLBWI and its dynamics during 1 µs of MD are largely consistent with a recently available Cryo-EM model of the orthologous complex in P. aeruginosa42, which we discuss further in detail below.
To describe observed new interfaces in the context of previously defined domains, we define four periplasmic interaction regions in FtsQLBWI (Fig. 1C, dashed boxes). These include a Truss region that links FtsQ to FtsI via an extended β sheet, a Hub region that contains a dense interaction network on both sides of FtsL and FtsB helices, a Lid region in which the anchor domain of FtsI interacts with FtsW ECL4 (extracellular loop 4 containing the putative catalytic residue FtsWD297,43), and a Pivot region where the first N-terminal periplasmic helix of FtsI interacts with FtsW ECL4. FtsI is involved in all four regions, contacting FtsL and FtsB in the membrane-distal periplasmic space and FtsW in the membrane, but essentially has no interaction with FtsQ. These four regions are rich in residues that, when mutated, give rise to superfission (SF) or dominant negative (DN) phenotypes, suggesting that they modulate FtsWI activity. While our analysis below focuses on a subset of residues and protein–protein interfaces with well-established phenotypes, we provide trajectories of top-ranked inter-residue hydrogen-bonding frequencies for each interface pair in the complex (Supplementary Data 1).
A collapsed structural model of FtsWI in the absence of FtsQLB reveals critical interactions in the Pivot region
Our FtsQLBWI model and FtsW, FtsN, and FtsB single-molecule tracking results suggest that FtsQLB forms a complex with FtsWI on the Z track that is poised for further activation by FtsN on the sPG track. To investigate this possibility, we performed an MD simulation of an FtsWI system without FtsQLB. In this simulation we observed flexibility in FtsI periplasmic domains in the absence of FtsQLB. FtsI rotated about the Pivot region and collapsed onto the membrane, where it remained until the end of the 1 µs simulation (Fig. 1D and Movie S4). While 1 µs is insufficient to sample all conformations of FtsI in equilibrium, a collapsed FtsI conformation is consistent with various flexible conformations of the paralogous Thermus thermophilus PBP2 in the elongation complex with RodA in a previous cryo-EM study39. Notably, FtsI tilts in the opposite direction relative to FtsW in comparison to a RodA-PBP2 crystal structure, with E. coli FtsI missing a small loop that mediates RodA-PBP2 interaction in T. thermophilus PBP2 (Fig. S5C).
In the FtsQLBWI structure, the Pivot region consists of an FtsI helix (FtsID51–S61) on top of the second short helix (FtsWS260–G274) of FtsW ECL4 and is maintained by hydrogen bonding between FtsIR60-FtsWQ266 (Fig. S6A) and hydrophobic contact between FtsIL53 and FtsWM269 (Figs. 1E and S7A). These contacts were broken in FtsWI in the absence of FtsQLB, resulting in separation of FtsIL53 and FtsWM269 and of FtsIR60 and FtsWQ266 in the FtsWI trajectory (Figs. 1F, S6B, and S7B). Previous work showed that mutations to charged residues in this interface (FtsIG57D, FtsWM269K) lead to the failure of cell wall constriction, while replacing with a hydrophobic residue (FtsWM269I) produced a SF variant of FtsW20,21,44. As the Pivot region conformation is impacted by distal interactions with FtsQLB and contains both DN and SF mutations, it is likely key for regulating FtsWI activity.
A β-sheet formed by the extreme C-termini of FtsQLBI in the Truss region stabilizes an extended conformation of FtsI
To identify what specific interactions between FtsI and FtsQLB support the extended conformation of FtsI periplasmic domains, we examined the Truss region. The Truss region consists of the C-terminal domains of FtsQLB and FtsI, with a striking feature that each one of the four proteins contributes one β-strand to extend an FtsQ β-sheet (Figs. 1C and 2A). The β-sheet formed between FtsQA252–A257 (β12) and FtsBT83–P89 (β1) has been previously reported45 (Fig. S5B), but that between FtsLN116–Q120 (β1) and FtsIE575–I578 (β16) has not been observed experimentally and was only recently identified by structure prediction46. FtsL indirectly interacts with FtsQ via FtsB within this β-sheet. The β-strand of FtsLN116–Q120 maintains β dihedral angles throughout the 1-μs simulation, while FtsIE575–I578, which terminates the β-sheet, exhibits greater flexibility (Fig. S8A, B). Previously, it was reported that the FtsI C-terminus (FtsII578–S588) was cleaved, although it was unclear whether the cleavage occurred post-translationally, or during sample processing47,48. We performed an additional 200-ns MD simulation of FtsQLBWIΔI578–S588 and observed that this truncation did not disrupt β-strand formation of FtsLN116–Q120 or FtsIE575–V577 (Fig. S8C, D). In addition to the β-sheet linking these four proteins, two important contacts were observed between FtsIR559 and FtsLE115, and between FtsIR239 and FtsQD249, which may strengthen C-terminal interactions between FtsI and FtsQLB (Figs. S9, S10, and S11).
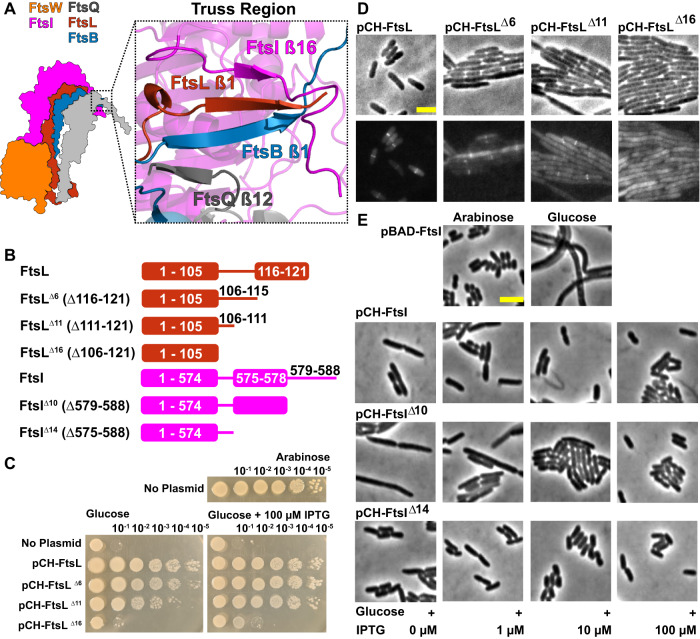
Fig. 2. C-terminal extended β-sheet of FtsQLBI in the Truss region is important for cell division.
A A detailed view of the Truss region in the final frame of the FtsQLBWI 1-μs MD simulation illustrates β-sheet interactions between the C-terminal ends of FtsQ (gray), FtsB (blue), FtsL (red), and FtsI (magenta). B Cartoon showing FtsL and FtsI β-strand truncation mutants. Also see Fig. S12 for details. C Spot dilution complementation test of FtsL truncation mutants. E. coli cells depleted of chromosomal wild-type FtsL but contain FtsL expressed from the PBAD promoter (strain MDG279, Table S2) complemented in the presence of arabinose (top panel), but failed in the presence of glucose (No Plasmid, first rows of the bottom panel). The same depletion strain (MDG279) expressing wild-type FtsL (pCH-FtsL, or pBMB064), FtsLΔ6 (pCH-FtsLΔ6, or pBMB065) and FtsLΔ11 (pCH-FtsLΔ11, or pBMB066, Table S1) from a lac promoter on plasmids complemented the depletion in the presence of glucose at both no induction and 100 μM IPTG conditions (middle rows of both panels). pCH-FtsLΔ16 is unable to complement at both conditions (bottom rows of the bottom panel). See Fig. S13 for more induction conditions. D Images of E. coli cells depleted of wild-type FtsL and expressing an mVenus fusion to FtsL of various truncations showed that truncations of FtsL of increasing length exhibited increasing cell length (top) and decreased FtsL midcell localization (bottom) relative to cells expressing mVenus fused to full-length FtsL. Scale bar 3μm. See Fig. S13 for quantifications. E Images of E. coli cells depleted of wild-type FtsI and expressing FtsI truncations. A wild-type FtsI fusion and FtsIΔ14 exhibit near-normal cell lengths even at low induction levels, while FtsIΔ11 exhibits filamentous cells at low expression levels. Scale bar 3 μm. See Fig. S14 for quantifications. Source data are provided as a Source Data file.
To verify the importance of the β-sheet formed between FtsL and FtsI, we constructed three FtsL mutants with varied degrees of C-terminal truncations (FtsLΔ6, FtsLΔ11, and FtsLΔ16). All three lack the C-terminal β-strand, and FtsLΔ11 and FtsLΔ16 additionally lack residues such as FtsLE115 that interacts with FtsI (Figs. 2B and S12). FtsLΔ6 and FtsLΔ11 cells were filamentous, indicating a division defect, but complemented an FtsL depletion strain at the highest induction level tested (100 μM IPTG). FtsLΔ16 cells remained filamentous and failed to rescue cell division under the same condition (Figs. 2C, S13A, B, and Table S6). Fluorescently labeled FtsLΔ6 and FtsLΔ11 (mVenus-FtsL) fusions showed clear, but significantly reduced, midcell localization, while mVenus-FtsLΔ16 cells exhibited only diffusive, cytoplasmic fluorescence (Figs. 2D, S13C, Tables S7, and S8). These different localization patterns were not due to mVenus-FtsL truncations’ differential expression levels or their abilities to support cell division (Fig. S13D, E). These results suggest that the interactions of the C-terminal β-strand of FtsL with FtsB and FtsI are important for the recruitment of the complex, while further truncations impact essential divisome functions. They are consistent with a previous observation that a C-terminal truncation mutant, FtsLΔ114–121 (or FtslΔ7), complemented FtsL depletion, but was defective in co-immunoprecipitating with FtsQ49 and produced wrinkled colonies50.
Next, to investigate the role of β-strand interaction between FtsL and FtsI, we constructed two FtsI C-terminal truncations, FtsIΔN579–S588 (or FtsIΔ10), where only the disordered C-terminus after the predicted β-strand was deleted, and FtsIΔE575–S588 (or FtsIΔ14), where the β-strand and the C-terminus were both deleted (Figs. 2B, S12, and Table S9). We note that reported post-translational cleavage of FtsI between FtsII578–S588 would be FtsIΔ11 following this nomenclature, removing one residue with a hydrophobic sidechain from the predicted β-strand that interacts with FtsL. Both FtsIΔ10 and FtsIΔ14 complemented FtsI depletion (Fig. S14A). FtsIΔ10 cells were longer (l = 5.6 ± 1.3, μ ± s.e.m. calculated from two biological replicates for n = 446 total cells) than wild-type cells (l = 3.9 ± 0.0, μ ± s.e.m., n = 1215 cells) at the minimum expression level (1 μM IPTG). However, extending the truncation in FtsIΔ14 corrected this defect, resulting in cell lengths marginally shorter than wild-type (l = 3.3 ± 0.0, μ ± s.e.m., n = 487 cells) at the minimum expression level (Figs. 2E, S14B, and Table S9). Western blot showed that FtsIΔ10 expression was lower than that for either FtsI or FtsIΔ14 at equivalent induction levels, and that FtsIΔ14 expression was marginally higher than that of FtsI (Fig. S14C, D and Table S10). Little change was observed in midcell localization of FtsI across all conditions (Fig. S14E and Table S11). Together, these observations indicate that interactions between C-terminal β-strands of FtsI and FtsL may modulate FtsI stability and/or FtsWI activity.
Hub region reveal inhibitory and activating interactions between FtsL and FtsI
The Hub region lies beneath the Truss region, encompassing the previously identified CCD interface (Constriction Control Domain, containing multiple SF residues9,10,51) on one side of FtsL and FtsB helices (Figs. 3A and S15), and the AWI interface (Activation of FtsW and FtsI, containing multiple DN residues52) on the other side (Fig. 3B). As such, the Hub region may be a key regulatory region for the activities of FtsWI. We analyzed predicted interactions in the Hub region that were maintained during the final 500 ns of a 1 µs simulation of the FtsQLBWI system, focusing on hydrogen bonds (as defined in “Methods”) and hydrophobic contacts by computing the distance between the side chain geometry center of each residue in a given pair.
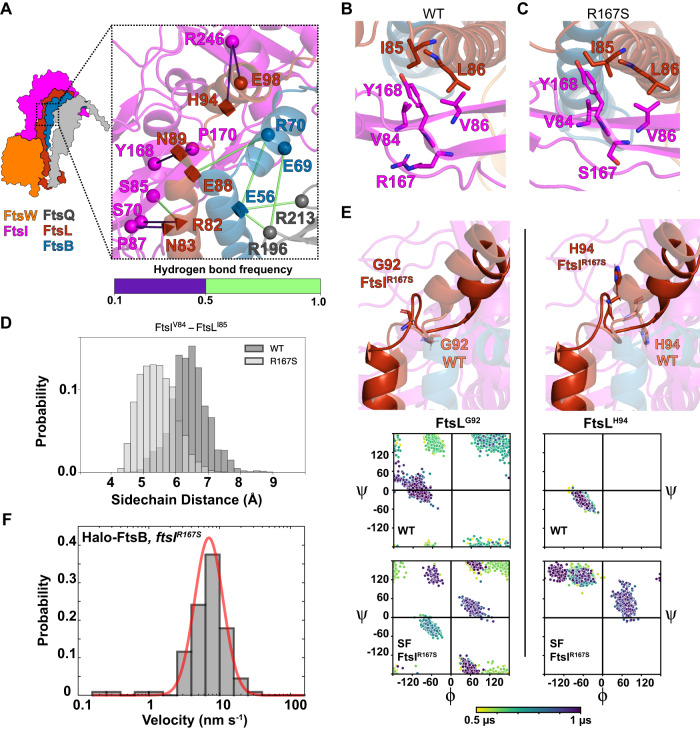
Fig. 3. Inhibitory and activating interactions between FtsL and FtsI in the Hub region are distributed along the CCD (A) and AWI interfaces (B and C).
A A hydrogen-bonding network extends from FtsQLB to FtsI in the CCD interface of the Hub region. Shapes indicate residues involved in hydrogen bonding. Superfission mutant residues (FtsLN89, FtsLE88, FtsLH94, and FtsBE56) are depicted as squares. Dominant negative mutant residues (FtsLR82 and FtsLN83) depicted as tetrahedrons. Spheres indicate other residues involved (FtsIP87, S70, S85, Y168, P170, R246, FtsLE98, FtsBE69, R70, and FtsQR196, R213). Hydrogen-bond frequencies during the last 500 ns of FtsQLBWI simulation are indicated by the color bar. B Hydrophobic packing among FtsLI85, FtsLL86, FtsIV86, FtsIV84, and FtsIY168 is observed in the AWI domain between FtsL (red) and FtsI (magenta) in the WT FtsQLBWI simulation. C The same AWI interface exhibits a tighter hydrophobic packing in the FtsQLBWIR167S complex simulation. Note the relative differences between FtsIV84,Y168 and FtsLI85. D The distance between the centers of geometry of FtsIV84 and FtsLI85 sidechains decreased from d = 6.4 ± 0.6 Å in the WT FtsQLBWI complex to 5.4 ± 0.5 Å in the FtsQLBWIR167S in the last 500 ns of simulations, consistent with the closer packing between the two residues in the SF FtsQLBWIR167S complex. E Top: conformations after 1 µs MD of FtsLG92 (left) and FtsLH94 (right) in the WT FtsQLBWI (tan color) and the SF FtsQLBWIR167S (dark red color) complexes show disruption of the second short α-helix of FtsL in the FtsQLBWIR167S complex. Bottom: dihedral angles of both FtsLG92 (left) and FtsLH94 (right) are disrupted in the FtsQLBWIR167S complex. A shift for FtsLH94 dihedral angles was observed from being α-helical (bottom left quadrant) for WT FtsQLBWI to being β-strand-like in FtsQLBWIR167S (top left quadrant). Dihedral angles are color-coded from yellow to dark purple in time in the 1 μs MD trajectories. F Single-molecule tracking of Halo-FtsB in a strain expressing FtsIR167S shows that Halo-FtsB molecules only exhibit the slow-moving population. The velocity histogram (gray bars) is best fit by a single population (red, vslow = 8.0 ± 0.4 nm/s, μ ± s.e.m., n = 112 segments). Source data are provided as a Source Data file.
The most prominent feature in the CCD interface of the Hub region is a network of residues with polar sidechains extending through FtsQLB to FtsI. As shown in Fig. 3A, contacts between FtsQR196-FtsBE69, FtsQR196-FtsBE56 and FtsQR213-FtsBE56 connect FtsQ to FtsB. FtsB is connected to FtsL through FtsBE56-FtsBR70 and FtsBR70-FtsLE88. Finally, FtsL connects to FtsI through FtsLR82-FtsIS85 (Figs. 3A and S16). Mutations of residues in this region such as FtsBE56A, FtsLE88K, FtsLN89S, FtsLG92D, and FtsLH94Y have been reported to be SF variants; cells expressing these variants are shorter than wild-type cells and able to survive in the absence of FtsN10,15. Therefore, it is possible that these interactions maintain FtsI in an inactive conformation, and that abolishing these inhibitory interactions activates FtsI.
Interactions in the AWI interface are mainly between one face of the FtsL helix and two β-strands at the neck of the FtsI head domain (Fig. 3B). A few hydrophobic residues, FtsIY168, FtsIV84, and FtsIV86, pack closely with hydrophobic residues FtsLI85 and FtsLL86. Additionally, hydrogen bonds were observed between FtsLR82-FtsIS85 and between FtsLN83-FtsIP87 (Fig. S16, hydrogen-bond frequencies for all protein–protein interfaces in the complex are available in Supplementary Data 1). Previously both FtsLR82E and FtsLL86F were identified as DN mutants 10, suggesting that these interactions may be required for maintaining the active conformation of FtsI.
MD simulation of the FtsIR167S SF variant suggests an active conformation of FtsI
We reasoned that if predicted inhibitory interactions in the CCD region and activating interactions in the AWI region play important roles in modulating FtsI activity, we may observe corresponding conformational changes in the Hub region when some of these residues are mutated. To examine interactions in the AWI region, we performed a 1-μs MD simulation of a SF variant, FtsIR167S17 (Fig. S17A). FtsIR167 is next to hydrophobic residues FtsIY168, FtsIV84, and FtsIV86 that interact with FtsLI85 and FtsLL86 (Fig. 3B). In the FtsIR167S simulation, the two β-strands at the neck of the FtsI head domain pack closer to FtsL than in the WT (FtsQLBWI) simulation, as measured by the sidechain geometry center distances between FtsIV84 and FtsLI85 (Figs. 3C, D, and S18A, B; d = 6.4 ± 0.6 Å for WT and 5.4 ± 0.5 Å for FtsIR167S complex). Consistent with this measurement, the solvent-accessible surface area of FtsL residues with hydrophobic sidechains in the helix linking the Lid and Hub regions was reduced from 534 Å2 to 501 Å2.
Interestingly, we observed drastic conformational changes in the turn connecting the two FtsL helices immediately above the AWI region of the Hub. As shown in Fig. 3E, this conformational change corresponds to a transition in the distribution of dihedral angles for FtsLG92 and with FtsLH94 shifting from α-helical to β-strand dihedral angles, while the WT complex retains α-helical dihedral angles. Note that both FtsLH94Y and FtsLG92D are SF variants, suggesting that the conformation of this region plays an important role in FtsWI regulation. Taken together, the FtsIR167S SF complex simulation suggests that replacing the charged arginine residue with uncharged serine in FtsIR167S strengthens hydrophobic interactions between FtsI and FtsL in the AWI region and results a conformational change in FtsL helices. These changes may correspond to an activated conformation of FtsWI, leading to the SF phenotype of FtsIR167S.
FtsB remains associated with FtsWI in the FtsIR167S SF background
In our previous single-molecule tracking experiments, we observed that FtsWI shifts to the slow-moving, active population on the sPG track in the FtsBE56A, FtsIR167S, and FtsWE289G SF backgrounds17. We asked whether activation of FtsWI was due to the dissociation of FtsQLB from FtsWI on the Z track to relieve an inhibitory effect as previously proposed19, or if FtsQLB remains in complex with active FtsWI on the sPG track to maintain its activities as recently suggested by an in vitro study9. To distinguish between these two possibilities, we performed single-molecule tracking of Halo-FtsB in the FtsIR167S SF background under a rich growth condition, which was previously used to assess the effect on FtsW. As shown in Fig. 3F, we observed that Halo-FtsB was best fit as a single, slow-moving, active population (vslow = 8.0 ± 0.4 nm/s, μ ± s.e.m., n = 112 segments, Table S3), just as we previously observed for FtsW. This result is consistent with the expectation that FtsIR167S enhances the formation of the active FtsQLBWI complex on the sPG synthesis track, and that FtsQLB remains in the complex with activated FtsWI, as we previously reported for FtsN18.
Lid region reveals important interactions between FtsI anchor domain and FtsW ECL4 that may modulate FtsW activity
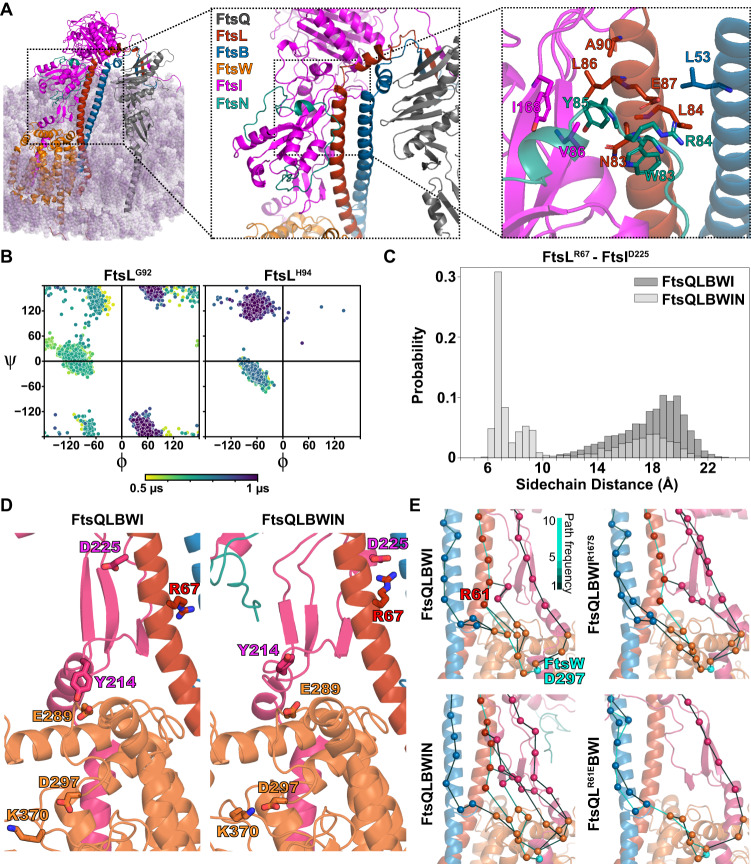
The Lid region is located at the periplasmic face of the inner membrane and involves interactions between a polar patch on the back of FtsL and FtsB24, a loop in the FtsI anchor domain (FtsI anchor-loop) and FtsW ECL4 (Figs. 4A, S19, and S20). Specifically, hydrogen bonds between FtsBK23-FtsIE219, FtsBD35-FtsLR67, FtsLR67-FtsID220, and FtsLE68-FtsIR207 persist during the final 500 ns of FtsQLBWI simulation and fix the orientation of the FtsI anchor-loop with respect to the FtsL and FtsB helices (Figs. 4A and S19), while hydrogen bonding between FtsLR61, FtsWN283, and FtsWS284 positions ECL4 directly beneath the anchor-loop (Figs. 4B and S20). These interactions allow FtsIY214 in the FtsI anchor-loop to hydrogen bond with FtsWE289, positioning FtsW ECL4 beside a central pore containing the putative catalytic residue FtsWD297 20 (Figs. 4B, C, and S20). Previous studies showed that FtsWE289G is a SF variant while FtsLR61E and FtsLR67E are DN variants24, indicating that altering the interactions in the Lid region can indeed render a constitutively active or inactive complex.
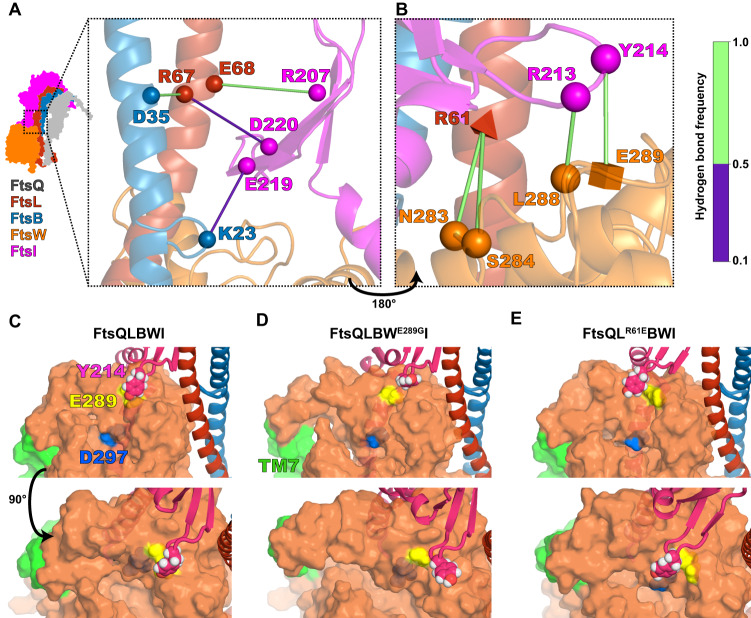
Fig. 4. Interactions between FtsB, FtsL, FtsI, and FtsW position the FtsI anchor-loop near the FtsW active site; FtsI anchor-loop position is correlated with FtsWI activity.
A, B Hydrogen bonding in the Lid region. Shapes indicate residues with hydrogen bonding during the last 500 ns of FtsQLBWI simulation, with a cube specifying SF mutation FtsWE289G, a tetrahedron specifying DN mutation FtsLR61E, and spheres indicate other residues involved (FtsIR207, R213, Y214, E219, D220, FtsLR67, E68, FtsBK23, D35). Hydrogen-bonding frequencies during the last 500 ns of the simulation are indicated by the color bar. Two views of the Lid region are shown: A interactions between the FtsI anchor domain (magenta), FtsL (red), and FtsB (blue), which position the anchor domain above FtsW ECL4; B the same region rotated ~180° to show how interactions between residues in ECL4 of FtsW (orange) with FtsLR61, FtsIR213, and FtsIY214 position FtsW ECL4 (orange) below the FtsI anchor-loop (magenta). C–E Side and top views of a cavity on the periplasmic face of FtsW (orange) containing the putative catalytic residue FtsWD297 (blue) lying below the FtsWE289 residue (yellow; D shows SF FtsWE289G) for WT FtsQLBWI (C), SF variant FtsQLBWE289GI (D) and DN mutant FtsQLR61EBWI (E) complexes following 1 µs MD simulations. With SF mutation FtsWE289G, the FtsI anchor-loop (magenta) including FtsIY214 (pink and white spheres) moves away from the cavity, which is expanded as FtsW TM7 (green) tilts into the bilayer. With DN mutation FtsLR61E, the anchor-loop (magenta) moves and FtsIY214 moves over the cavity, which is stabilized by an interaction between FtsIR216 and FtsWE289. Source data are provided as a Source Data file.
Observing that Lid region interactions in FtsQLBWI are rich in SF and DN residues, we hypothesize that local conformational changes resulting from these mutations could shed light on the FtsW activation mechanism. We simulated the effects of introducing the FtsWE289G SF variant and the FtsLR61E DN variant into FtsQLBWI. Both complexes adopt similar global structures after 1 μs of MD compared to WT FtsQLBWI (Fig. S17B, C), but differ in local positioning of the FtsI anchor-loop relative to FtsW ECL4 (Fig. 4D, E). In the SF FtsWE289G complex, the FtsI anchor-loop is rotated sideways toward FtsL and FtsW TMH1, opening a central cavity of FtsW (Fig. 4D). This conformation is stabilized by backbone hydrogen bonding between FtsIR213 and FtsWL288 in addition to maintaining contacts with FtsL (Fig. S20). In contrast, in the DN FtsLR61E complex, the FtsI anchor-loop is directly above the FtsW cavity (Fig. 4E). This interaction is stabilized by contacts between FtsIR216 and FtsWE289 and persists through ~72% of the last 500 ns of simulation of the FtsLR61E DN complex (Fig. S21). As we described above, the FtsI anchor-loop is coordinated by its interactions with the polar patch on one side of FtsL helix and with FtsW ECL4, which are reduced in the FtsLR61E variant (Fig. S17C). Loss of the interaction between FtsLR61E and FtsIE219 is also accompanied by conformational changes in FtsI; the relative orientation of FtsI and FtsW helices in the Pivot region changes, and the β-sheet in the anchor domain tilts towards FtsL (Fig. S17C). We also observed that in the SF FtsWE289G simulation FtsW TM7 tilts outward and expose a large extracytoplasmic cavity (Figs. 4D and S17B) as reported for the RodA-PBP2 complex39. In simulations of both FtsWI in the absence of FtsQLB and FtsQLBWI with FtsLR61E, FtsWY379 in ECL5 flipped from its predicted position to fit between FtsW TM1 and TM2. This flip was only observed in simulations of these inactive complexes, suggesting that FtsW-FtsL interactions may influence conformation near the FtsW active site.
Binding of FtsNE reduces inhibitory interactions and strengthens activating interactions in the FtsQLBWI complex
Previous studies have shown that the effects of SF variants are independent of FtsN, i.e. SF variants bypass the need for FtsN binding. Therefore, we reason that binding of FtsN to FtsQLBWI could switch the complex into an active conformation similar to what we observed for SF variants. To investigate the role of FtsN binding, we predicted the structure of FtsQLBWI with the addition of FtsNK58–V108, which encompasses FtsNE (FtsNL75–Q93)15 and a sufficient number of surrounding periplasmic residues required to obtain a predicted structure. As with other complexes, we performed 1 μs of MD to investigate interactions and dynamics of the predicted complex of FtsQLBWI and FtsNK58–V108 (FtsQLBWIN, Movie S5).
While termini of FtsNK58–V108 did not form stable interactions on this timescale, the FtsNE region is bookended by two interfaces predicted with high confidence and stability in MD (Fig. 5A). First, FtsNL75–P79 forms a polyproline II helix that binds the FtsI head domain (Fig. S22A). This region consists of residues with high polyproline II helix propensity53, which is a property conserved for FtsN (Fig. S22B) in taxonomic families within the previously identified subgroups characterized by a four-amino-acid deletion in RpoB54,55. However, we did not identify this motif for FtsN outside of these subgroups (e.g. in Pseudomonadaceae) and predicted complexes with both P. aeruginosa FtsI and PBP3x did not include this interaction (Fig. S22C), suggesting that FtsN interactions and functions may vary between γ-proteobacteria. This observation is consistent with the conditional essentiality of FtsN and the inability to detect an effect of FtsN on FtsQLBWI or reconstitute FtsQLBWIN in vitro for P. aeruginosa9,42.
Fig. 5. FtsNE binding reduces inhibitory interactions and induces conformational changes observed in SF complexes.
A Conformer of the FtsQLBWIN complex after 1 µs MD, with FtsNE shown in teal and detailed views of FtsN binding in the Hub region. Highly conserved and essential residues of FtsN, FtsNW83, FtsNR84, and FtsNY85 are at the AWI interface in the Hub region, close to residues previously identified in the FtsIR167S SF variant complex, including FtsIV86, I168 and FtsLL86, N83, A90, E87, L84. B Dihedral angle analyses of FtsLG92 (left) and FtsLH94 (right) in the FtsN-bound complex showed similar backbone dihedral angle changes compared to those in the SF mutant FtsQLBWIR167S (Fig. 3E). C The distribution of distances between sidechain centers of geometry for FtsLR67 and FtsID225 in the FtsQLBWIN simulation (light gray) exhibited a second, decreased peak compared to that in the FtsQLBWI simulation (dark gray). The distance decrease in FtsQLBWIN occurred with a conformational change in the last 300 ns of the MD simulation (trajectory in Fig. S23), demonstrating tighter packing between the FtsI anchor domain and FtsL in the presence of FtsN. D Interaction between the FtsI anchor-loop residue (magenta) FtsIY214 and FtsWE289 (orange) is disrupted in FtsQLBWIN as FtsID225 in the anchor domain increased its interaction with FtsLR67. This interaction moves the sidechain of FtsIY214 (magenta) away from the putative catalytic residue FtsWD297 (orange), similar to what was observed in the SF complex FtsQLBWE289GI (Fig. 4D). Note that FtsWK370 forms a salt bridge with FtsWD297 in FtsQLBWIN, but not FtsQLBWI. E Ensembles of optimal paths calculated from FtsQ to FtsWD297 (teal) showing the 10 most optimal paths in the final 500 ns of MD for FtsQLBWI (top left), FtsQLBWIR167S (top right), FtsQLBWIN (bottom left), and FtsQLR61EBWI (bottom right). Line colors correspond to the number of paths connecting pairs of residues in the ensemble (colorbar). The DN variant FtsLR61E (bottom right) eliminated all direct paths between FtsL to FtsW, shifting to paths through a small loop extending from the FtsB helix. Addition of FtsNE increased the density of paths through FtsI, which also includes paths through the Pivot region. Source data are provided as a Source Data file.
The second interaction region, FtsNW83–L89, contains conserved residues FtsNW83, FtsNY85, and FtsNL89, which are also the most sensitive residues to alanine scanning mutagenesis15. This region interacts with both the AWI interface of FtsL (FtsLL86, FtsLA90) and at the neck of the FtsI head domain (FtsIV86 and FtsIY168), largely through hydrophobic interactions and also including FtsBL53 (Fig. 5A, right). Importantly, the FtsL and FtsI residues bound by FtsN overlap with those impacted in the FtsIR167S SF variant (FtsLL86, FtsIV86, and FtsIY168), suggesting a shared mechanism of FtsQLBWI activation. Furthermore, this FtsN-binding interface includes both FtsLA90 in the hydrophobic interface and an FtsLE87-FtsNR84 interaction, suggesting that DN variants FtsLE87K and FtsLA90E are defective in FtsNE binding. The solvent-accessible surface area of FtsL residues with hydrophobic sidechains in this region was reduced from 534 Å2 in WT to 383 Å2, greater than that in the FtsIR167S simulation.
To validate the prediction of FtsN binding specificity, we additionally predicted structures of FtsNK58–V108 together with only FtsQLB or FtsWI. Despite relatively low prediction confidence, the predicted location of FtsNW83–L89 binding did not change (Fig. S22D). We also note that predictions of full-length FtsN are possible for E. coli and homologous complexes such as for Aliivibrio fischeri, but we failed to identify other high-confidence binding interfaces (Fig. S22E).
Little global change was observed in simulations of FtsQLBWIN compared to FtsQLBWI (Fig. S17D). However, MD revealed that the presence of FtsNE triggered extensive local conformational changes in all four interaction regions in FtsQLBWI. In the Truss region, the FtsLE115-FtsIR559 interaction was broken (Figs. S9A and S10E), which impacts interactions between the FtsL β-strand and adjacent FtsB and FtsI β-strands. Several interactions involving polar residues at the CCD interface (FtsLE98-FtsIR246 and FtsLN89-FtsIP170) in the Hub region were nearly completely abolished (Fig. S16), resulting in conformational change for FtsLH94 and FtsLG92 similar to that observed for FtsQLBWIR167S (Fig. 5B). In the Lid region, charged interactions between the polar patch of FtsL and the back of the FtsI anchor domain (FtsLR67-FtsID225, Figs. 5C and S23) are present; FtsWE289 in ECL4 switches from interacting with FtsIY214 to FtsIR213 in the anchor-loop, resulting in conformation changes in the FtsI anchor-loop and FtsW ECL4 that alter the catalytic cavity on the periplasmic face of FtsW relative to the conformer observed for FtsQLBWI (Fig. 5D). This conformational change, coincident with loss of FtsWE289-FtsIY214 interaction, is similar to that observed with the FtsWE289G SF mutation (Figs. 4D and S22F). However, the same conformational change did not occur in the FtsIR167S simulation within 1 μs and it remains unclear if this specific conformational change is necessary or sufficient for FtsW activation (Fig. S22G). In the FtsQLBWIN simulation, we also observed that FtsWK370 flipped to form a salt bridge with FtsWD297 within the first 200 ns that persisted to the end of the simulation (Fig. 5D, Movie S5). This was also observed in FtsIR167S and FtsWE289G SF simulations, but not in the wild-type FtsQLBWI simulation. The consistency between FtsNE-induced conformational changes and those that appeared in simulations of SF complexes with FtsIE289G or FtsIR167S, but not the wild-type FtsQLBWI or the DN FtsLR61E complex, suggests that FtsNE binding in the Hub region has allosteric effects on FtsW and/or FtsI activity in the FtsQLBWI complex.
Defining long-range interaction paths of FtsQLB complexes
Analyses described above allow us to identify critical local interactions that are modulated by FtsNE binding and may contribute to the activation of FtsWI, but do not reveal how local interactions trigger distal conformational changes in the complex. To investigate this, we compared long-range interaction pathways for wild-type and two active (FtsIR167S or FtsNE) complexes, as well as for the FtsIR61E DN complex. We quantified correlations between pairs of residues using a dynamical network model56. Highly correlated residues in a simulation trajectory of a complex reflect their coordinated motions, allowing us to construct long-range interaction pathways. Because FtsQ is primarily connected to the rest of the complex through its interactions with FtsB in the C-terminal Truss region, and because we wish to identify long-range interactions that affect the activities of FtsW and FtsI, we computed optimal paths through the Truss and Hub regions between FtsQ and putative catalytic residues FtsWD297,20 and FtsIS307 (Fig. S24). These paths reveal dominant routes of communication between the proteins. The optimal path ensemble between FtsQ and FtsWD297 for the DN FtsLR61E simulation exhibits greater path density through FtsB, while the other simulation systems exhibit greater path density through FtsL (Figs. 5E and S24A). Interestingly, active FtsIR167S and especially FtsNE simulations included more FtsI residues in optimal paths connecting FtsI to FtsW, which is consistent with our observation that FtsNE binds between FtsL and the FtsI head domain in the Hub region to enhance this interaction. The optimal path ensemble between FtsQ and FtsIS307 extends through SF residues including FtsBE56, FtsLE88, and FtsLH94, and varied across different complexes (Fig. S24B).
Comparison with a cryo-EM model of the orthologous complex in Pseudomonas aeruginosa
The recent publication of the Cryo-EM structure of the FtsQLBWI complex in P. aeruginosa42 makes it possible to address concerns about the physiological relevance of molecular dynamics of predicted divisome structures in E. coli. We found that the global conformations of the E. coli FtsQLBWI complex was remarkably similar to the P. aeruginosa complex (Fig. S25A). The P. aeruginosa cryo-EM structure was reported to exhibit a tilt in its FtsI TPase domain, relative to an AF2-predicted structure. We observed a similar tilt of FtsI early in all simulations and measured an angle of only 3° between the P. aeruginosa cryo-EM structure and the E. coli FtsQLBWI complex after 1 µs MD. Rapid tilt of the TPase domain towards the membrane was observed within the first 200 ns of MD for simulations of all E. coli complexes (Fig. S25B). Furthermore, Käshammer et al. described a change in the interface between the FtsI anchor domain and FtsL, where FtsL-interacting residues in the FtsI anchor domain shift from residues FtsI203–206 in the AF2 prediction to residues FtsI208–212 in the cryo-EM structure. We observed a similar conformational change as an interface is formed between FtsI223–228 (equivalent to P. aeruginosa FtsI209–215) and FtsL69–75 upon the addition of FtsNE (Fig. S25C). Unlike in the P. aeruginosa structure, we did not observe a direct interaction between the FtsI head and anchor domains. Instead, the conformer after 1 µs MD for the E. coli complex with FtsN suggests that FtsN bridges the head and anchor domains. This may reflect differing roles and interactions for FtsN in E. coli and P. aeruginosa divisome.
Discussion
In this work, we first used single-molecule tracking to determine that E. coli FtsQLB forms a complex with both inactive FtsWI on the Z track and active FtsWI on the sPG track (Fig. 1A, B). Building upon this result, we used structure prediction and molecular dynamic simulations to model the structures of FtsQLBWI (Fig. 1C), FtsWI (Fig. 1D), and FtsQLBWIN (Fig. 5A). We then examined the structural models by a set of mutations both experimentally (Figs. 2C–E and 3F) and computationally (Figs. 3C–F, 4C, and 5D–E). Combining these structural models with observations from MD and in biological experiments, we propose a regulatory mechanism in which FtsWI activities depend on FtsQLB and are further activated by FtsN in E. coli.
In the absence of FtsQLB, FtsWI adopts flexible conformations with low GTase and TPase activities because FtsWI lacks interactions in the Pivot and Lid regions that stabilize FtsWI conformations required for processive sPG strand polymerization and crosslinking. The addition of FtsQLB stabilizes an extended conformation of FtsI through the Truss region. This conformation supports higher FtsWI activities compared to the background level of FtsWI alone, and could be sufficient in species that are not dependent on FtsN. The binding of FtsNE both weakens inhibitory interactions in the CCD interface and enhances activating interactions in the AWI interface of the Hub region, collectively resulting in stronger association of the FtsI head domain and FtsL. These changes promote conformations in which interactions between the FtsI anchor-loop and FtsW ECL4 are modified to open the catalytic pore of FtsW. We propose that this conformational change corresponds to the highest activities of the complex because it remodels the catalytic cavity of FtsW to facilitate processive PG polymerization and to allow the growing PG strand to reach the TPase domain of FtsI.
Further, it remains a possibility that binding of the nascent PG strand to FtsI may be coupled with a transient conformational change to an extended conformation compatible with PG crosslinking as proposed for the RodA-PBP2 elongasome complex39 and observed in our predicted structures (Fig. S3). This mechanism differs in specific details from those proposed in recent work that also drew from structure prediction22,42,46. Despite their differences, each of these hypotheses involves allostery linking periplasmic interactions to distal active sites.
Roles of FtsQLB in FtsWI activation
The structural models of various complexes allowed us to clarify the seemingly contradictory roles of FtsQLB in activating FtsWI. Using a thioester substrate of FtsI, Boes et al., found that adding purified E. coli FtsQ moderately inhibited FtsI TPase activity, while adding FtsL and FtsB had little effect19. Marmont et al., found that purified P. aeruginosa FtsL and FtsB enhanced FtsW GTase activity as well as FtsQLB9. In neither case did the addition of FtsN impact FtsI or FtsW activity. Our model of FtsQLBWI shows that FtsQ has few interactions with members of the complex other than FtsB. Thus, a role of FtsQ is to stabilize the global conformation of the complex via these interactions without directly impacting the activity of FtsW or FtsI. The major role of FtsL and FtsB is to scaffold FtsWI in a conformation poised for activation by clamping both the membrane-distal TPase domain of FtsI and membrane-embedded FtsW. Truncations of β-strands in FtsLΔ6, FtsLΔ11, FtsLΔ16, and FtsIΔ10 were not lethal, but resulted in filamentous phenotypes indicating defects in cell division. Stacked β-strands between FtsL, FtsB, and FtsQ are consistent with the observation that C-terminal truncation of FtsLΔ100–121 (or FtsLΔ21) both abolishes FtsL interaction with FtsQ and also results in a reduced level of full-length FtsB49. A mild cell division defect was observed for FtsIΔ10 but not for FtsIΔ14, which is likely caused by a lower expression level of FtsIΔ10 than FtsIΔ14 under the same induction levels (Fig. S14). These observations suggest a complex role for C-terminal FtsL-FtsI interaction in the Truss region and are consistent with the observation that deletion of FtsIG571–V577 appears to be fully functional57. Note that our FtsB single-molecule tracking data indicates that FtsB is associated with FtsWI on both the Z track and the sPG track in E. coli, and hence whether an FtsWI complex alone is physiologically relevant is unclear.
The proposed activation mechanism is consistent with previous studies
The proposed activation mechanism is remarkably consistent with what was previously deduced by genetic studies: following recruitment by FtsQ, the activating signal goes from FtsB to FtsL, then to FtsI, and finally to FtsW10. We suggest that the final activation step includes remodeling the central cavity of FtsW to facilitate processive PG polymerization and crosslinking. This step can be achieved by multiple means: SF variants in the Pivot (such as FtsWM269I), Hub (such as FtsBE56A, FtsLH94Y, and FtsIR167S), and Lid (such as FtsIK211I) regions can correctly position the FtsI anchor domain away from the FtsW pore, or directly remodel the structure defined by the FtsI anchor-loop and FtsW ECL4 to open a channel in FtsW (such as FtsWE289G). However, short circuiting this pathway removes potential points of regulation needed to coordinate cell wall synthesis in space and time and in response to environmental conditions.
This activation mechanism is also consistent with previous mutagenesis studies, as our results provide unprecedented details in potential conformational changes caused by previously identified SF and DN mutants. For example, previously it was observed that substitutions removing the negative charge of FtsWE289 do not affect the function of FtsW while only FtsWE289G is superfission20. This observation can be explained by the fact that the loss of the side chain of FtsWE289 and/or the flexibility of FtsWG289, but not necessarily specific interactions, removes the capping of the FtsW cavity and activates FtsW GTase activity. The observation that SF variant FtsWE289G or FtsWM269I, but not overexpression of FtsN, can rescue the double-DN mutant FtsLL86F/E87K,10 is also consistent with our proposed activation mechanism and results. Since the double-DN mutant FtsLL86F/E87K loses its ability to bind to FtsN to trigger conformational changes that increase FtsWI activity via allosteric paths extending from the Hub through the Lid and Pivot regions (Fig. 5E), it is expected that Lid and/or Pivot mutations could short-circuit this effect.
The activation mechanism we propose does not specifically involve FtsWI catalytic residues. However, this model could be expanded to address possible regulation of active site conformation and dynamics near catalytic residues such as FtsWD297,20. For example, dynamics in the vicinity of FtsWD297 revealed potential roles of FtsWK370 in addition to FtsWY379 in regulating FtsW activity (Fig. 5D). In predictions, FtsWK370 blocks a putative substrate channel39, suggesting that FtsWK370 conformation can regulate substrate or product transport. We also did not address in detail the hypothesis of a diprotomeric Fts[QLBWI]2 complex or the role of cytoplasmic FtsL-FtsW interactions46. Nevertheless, we observed stable FtsL-FtsW cytoplasmic interaction in our simulations even though the N-terminal cytoplasmic tail of FtsW was truncated. Additionally, the location of the FtsNE binding site and conformational changes in FtsL and FtsB helices observed in simulations with FtsNE and FtsIR167S are qualitatively compatible with the diprotomer model that requires flexibility in this region.
Our simulations for all FtsQLBWI wild-type and mutant complexes, with and without FtsN, rapidly attained a bent conformation similar to that observed for the P. aeruginosa cryo-EM complex (Fig. S25B). Käshammer et al., hypothesized that a transition from the observed bent conformation to the AF2-predicted straight conformation could be associated with binding of substrate or proteins such as FtsN. Our results here, when further combined with our previous single-molecule imaging results for FtsN18, demonstrate that FtsNE is associated with FtsQLBWI on the sPG track, with MD suggesting that FtsN activates the GTase activity of FtsW in the observed bent conformation. We did not observe stable straight conformations in our simulations. Our model also provides insight into conflicting reports about the role of FtsN in different organisms. We observed that a shift in FtsI-FtsL interactions, analogous to that observed between predicted and experimental structures in the P. aeruginosa cryo-EM study, only occurred upon the addition of FtsNE in the E. coli complex (Fig. S25C).
This study comes with several limitations. Importantly, the absence of direct measurements of FtsN-FtsQLBWI binding, and simultaneous GTase and TPase activities, restricts our ability to draw definitive conclusions about the roles of individual protein–protein interfaces. Subsequent work can test hypotheses drawn from our findings, leveraging opportunities such as a recently reported E. coli divisome complex with in vitro transglycosylase activity42. In light of these limitations, we primarily focused on residues at protein–protein interfaces with well-characterized phenotypes. The significance of many newly identified interactions remains to be investigated. Second, while our results show consistency with the P. aeruginosa cryo-EM structure, we cannot disregard potential influences of model-dependence and random effects. Equilibrium MD of 1 μs is insufficient to accurately quantify binding free energies or to confidently identify new interfaces; simulation techniques with enhanced sampling should be considered in future research. Lastly, our simulations exclude FtsW and FtsI substrates as well as divisome proteins known to impact cell-wall synthesis48. Filling in these aspects of this picture will enable a more direct correlation between divisome structure and dynamics with substrate binding and enzymatic mechanisms.
In summary, our results are not only consistent with past biochemical and genetic studies, but also shed light on the molecular details of active and inactive conformations of FtsWI. The approach we developed in this work—structure prediction followed by MD simulation with results informing experiments to test hypotheses arising from modeled structures—proved powerful in generating new insights into molecular interactions in the divisome.
Methods
Complementation assay
To ensure proper growth of ΔftsL and ΔftsI strains, arabinose was included during maintenance and preparation to induce expression of FtsL and FtsI, respectively, from the PBAD promoter. Cells were grown overnight at 37 °C in LB and 0.2% arabinose from a single colony. The following day, the saturated culture was reinoculated 1:1000 in fresh LB with 0.2% arabinose. Cells were grown in log phase at 37 °C to an OD600 of 0.5 for all conditions. Cells were then washed three times with 0.9% saline. They were then serial diluted in 0.9% saline and plated on LB plates containing either 0.2% arabinose or 0.4% glucose (to repress expression from the PBAD promoter) and IPTG as noted. Plates were grown at 37 °C overnight and then imaged.
Plasmid and strain construction
Plasmids used in this study (Table S1) were assembled by Polymerase Chain Reactions (PCR) amplifying insert and vector DNA fragments followed by In-Fusion cloning (Takara Biosciences, In-Fusion HD Cloning Kit). Oligonucleotides (Integrated DNA Technologies) used in PCR amplifications are described in Table S12. All plasmids were verified by DNA sequencing. After construction, electroporation was used to transform plasmids to create strains of interest (Table S2) under appropriate antibiotic selection. Depletion strains were maintained under arabinose induction to maintain wild-type phenotype.
Single-molecule tracking sample preparation, imaging, and data analysis
Prior to imaging, cells were grown to log phase at 25 °C in defined minimal M9 medium (0.4% glucose, 1x MEM amino acids, and 1× MEM vitamins, M9(+) glucose). Cells were incubated for 20 min with the addition of 50 nM Janelia Fluor 646 (JF646). Following labeling, cells were washed 3 times with M9(–) glucose (0.4% glucose and 1x MEM vitamins). Cells were placed onto a 3% agarose gel pad (M9(–) glucose), sandwiched with a coverslip, and enclosed within an FSC2 chamber (Bioptechs) for imaging. Cells were imaged after 30 min of equilibration on the microscope.
For experiments with fosfomycin, 200 µg/ml of Fosfomycin was added to the gel pad and to the cells following the final wash step. Cells were imaged 1 h after Fosfomycin addition. For experiments with rich, defined medium, EZDRM (Teknova) was used in place of M9 for the growth media, wash buffer, and gel pad.
HaloTag-FtsB tracking was performed on a home-built microscope as previously described17,30. Briefly, strains were imaged on an Olympus IX-71 microscope body using an UPLANApo 100XOHR Objective (NA1.50/oil) with a 1.6 x field lens engaged. JF646 was imaged with a 674-nm laser (Coherent) at an excitation power density of ~25 W/cm2. The exposure time was 500 ms and the imaging frame rate was 1 Hz.
Single-molecule data analysis was performed as previously described17,18,30. Briefly, positions of single molecules were determined using the ImageJ (1.53f51) plug-in, ThunderSTORM (1.3-2014-11-08)58. The remaining data analysis and postprocessing was performed using home-built MATLAB scripts available from the Xiao Laboratory GitHub repository (XiaoLabJHU)17. In short, using a nearest-neighbor algorithm, single molecules were linked to trajectories. Using the bright-field image as a visual guide, only trajectories near the midplane of a cell’s long axis or visible constriction sites were chosen to ensure the measurements were made on single molecules at the cell-division site. The true displacement of tracked molecules around the circumference of a cell is underestimated in 2D single-molecule tracking due to the cylindrical cell envelope. Therefore, the trajectories were unwrapped to one-dimension. Unwrapped trajectories were then segmented manually to determine stationary or processive movement. These procedures were described in detail in17.
The cumulative probability distribution of directional moving velocities was calculated for each condition and fit to either a single or double log-normal population:
| 1 |
where v is the moving velocity and P1 is the percentage of the first population. For a single population, P1 = 1. The parameters μ and σ are the natural logarithmic mean and standard deviation of the log-normal distribution. The average velocity was calculated using To estimate errors in parameter estimates, CDF curves were generated for 1000 bootstrapped samples and fit, with the standard deviations of parameter fits estimating 1 standard error of measurement (s.e.m.).
Bright-field sample preparation, imaging, and western blots
Cells were grown overnight at 37 °C in LB and 0.2% arabinose from a single colony. The following day, the saturated culture was reinoculated 1:1000 in fresh LB with the appropriate inducer and/or repressor (0.2% arabinose, 0.4% glucose, and/or ITPG as noted). Cells were grown to log phase at 37 °C. Cells were then washed three times with 0.9% saline with the appropriate inducer and/or repressor (0.2% arabinose, 0.4% glucose, and/or ITPG as noted). Cells were placed onto a 3% agarose gel pad (0.9% saline with the appropriate inducer) and/or repressor (0.2% arabinose, 0.4% glucose, and/or ITPG as noted), sandwiched with a coverslip, and enclosed within an FSC2 chamber (Bioptechs) for immediate imaging. Phase imaging was performed on a home-built microscope as previously described30. Briefly, phase imaging was performed on the same microscope as that used for single-molecule tracking experiment with condenser lamp illumination using a UPLANFLN 100X Objective (NA1.30/oil).
For western blots cells were grown under the same conditions and harvested via centrifugation. Samples were resuspended in 2x Laemmli Sample Buffer (Bio-Rad) and heated for 10 min at 95 °C before loading onto a Mini-PROTEAN TGX Precast Gel (4–15%; Bio-Rad). Electrophoresis, transfer to nitrocellulose and blot development followed standard procedures. The primary antibody was polyclonal rabbit anti-FtsI sera (1:50,000 dilution). The secondary antibody was HRP goat anti-rabbit (1:50,000 dilution; ThermoFisher). Chemiluminescence was detected using a ChemiDoc System (Bio-Rad).
Prediction of protein complex structures
We used AlphaFold223 as implemented in ColabFold33 using the LocalColabFold version and AlphaFold2 parameters available in September 2021. ColabFold uses the MMseqs2 server59 to obtain sequence alignments, greatly reducing local storage and computation requirements. Predictions were made on a Google Cloud Platform instance with an A100 GPU, taking approximately 1.5 h including sidechain relaxation. Template structures were not used, and predictions utilized 48 recycle steps. In preliminary work, we found that AlphaFold2 model 3, which was trained without templates, typically provided the best performance as measured by local pLDDT and global pTMscore metrics. Predictions from model 3 for FtsWI, FtsQLBWI, and FtsQLBWIN were used to generate MD systems. Model 5, which was trained similarly and produced similar predictions for γ-proteobacterial complexes, was later found to reproduce some additional reported interactions in other species and was used for B. subtilis and S. pneumonia in Fig. S2B. We did not use AlphaFold-multimer25 for these predictions, as its initial implementation resulted in structures with steric clashes that could not be directly used for building MD systems. We note that this has since been addressed and that later versions of AlphaFold-multimer give similar predictions of divisome complexes to what we report that typically lack clashes and are appropriate for MD. All structure predictions shown in the manuscript, as well as protein complex structures following 1 µs of MD, are available as supplementary data.
Conservation of FtsNE
We constructed sequence logos illustrating FtsNE conservation following previously reported methods applied to the E. coli FtsN cytoplasmic domain60. Sequences annotated as FtsN (TIGRFAM code TIGR02223) were downloaded from AnnoTree61 for Enterobacteriaceae (513), Pasteurellaceae (113), and Vibrionaceae (227). Sequences that were incorrectly annotated based on length or other features were manually removed. Multiple sequences alignments were constructed using MUSCLE62 and sequence logos for regions corresponding to E. coli FtsN73–95 were obtained using WebLogo 363. Only 10 FtsN sequences were identified for Pseudomonadaceae following this method, so we obtained 304 sequences homologous to P. aeruginosa FtsN68–118 in Pseudomonadaceae using blastp against the RefSeq Select proteins database, verifying that this also reproduced conservation observed for Enterobacteriaceae when using E. coli FtsN58–108 in the same way.
Equilibrium molecular dynamics simulation of FtsQLBWI and FtsQLBWIN complexes with superfission and dominant negative variants
Simulation systems for molecular dynamics were constructed from AlphaFold models of FtsWI, FtsQLBWI, and FtsQLBWIN including the following subunits and residues: FtsQ (20–276), FtsL (1–121), FtsB (1–103), FtsW (46–414), FtsI (19–588), and FtsN (58–108). Mutations of interest were made using the Mutagenesis Wizard of the PyMOL molecular visualization software.
All systems were embedded in a POPE membrane and solvated (see Table S5 for system dimensions) with 150 mM NaCl in TIP3P water64 using the CHARMM-GUI Membrane Builder. All systems were electrically neutral. N-termini of FtsQ, FtsW, FtsI, and FtsN were capped with the CHARMM ACE patch, and the C-terminus of FtsN was capped with the CHARMM CT3 patch. Equilibration was performed according to the CHARMM-GUI equilibration protocol involving a series of gradually relaxing sidechain and backbone restraints. After equilibration, 5 ns pre-production simulations were performed prior to simulation on the special-purpose supercomputer Anton 2 at the Pittsburgh Supercomputing Center.
Simulations on Anton 2 were performed using the CHARMM36m forcefield in an NPT ensemble at 310 K, 1 atm, and with a timestep of 2.5 fs. Bond lengths for hydrogen atoms were constrained using the M-SHAKE algorithm65. An r-RESPA integrator66 was used; long-range electrostatics were computed every 6 fs. Long-range electrostatics interactions were calculated using the k-space Gaussian split Ewald method67. Trajectories were written at an output frequency of 0.24 ns/frame. Each trajectory was unwrapped using the PBCTools plugin of VMD and aligned to the backbone atoms of FtsW (segid PROD) to facilitate comparison across complexes. Code for analyzing simulations was written using the MDAnalysis python package (1.0.0). Hydrogen bonds between relevant interfaces were computed using the HydrogenBondAnalysis function of MDAnalysis (v 1.0.0) in which a hydrogen bond is defined by a distance cutoff of 3.0 Å and an angle cutoff of 150°.
Calculation of structure parameters
Solvent-accessible surface area was calculated for all hydrophobic FtsL residues in the region of FtsL interacting with FtsB and the FtsI head and anchor domains (residues 62–86 inclusive of FtsL residues L62, L63, A65, L70, V71, L72, A76, L77, I79, W81, L84, I85, and L86) using the get_area command in PyMOL (version 2.5) with parameters dot_solvent, dot_density, and solvent_radius set to 1, 4, and 1.4, respectively. FtsI TPase domain tilt angles were calculated following methods applied to the P. aeruginosa FtsQLBWI structure42. Trajectories were aligned by minimizing RMSD for FtsW atoms, and the coordinates of for FtsIS307 were tracked in this reference frame. The tilt angle is defined as the angle between two vectors connecting atoms for FtsWD297 and FtsIS307 in the AF2-predicted structure and in frames of the trajectory. The P. aeruginosa structure was compared by aligning to E. coli FtsW using the default settings for the align command in PyMol (version 2.5) and analyzing coordinates for equivalent residues.
Dynamical network analysis for computing optimal paths
Dynamical network representations of the FtsQBLWI, FtsQLBWIR167S, FtsQLR61EBWI, and FtsQLBWIN complexes were generated using the dynetan python package (1.0.1)68. In the network, each node is defined as the atom of a residue, and pairs of nodes are considered connected if their heavy atoms are within 4.5 Å of each other for at least 75% of the final 500 ns of the trajectory. The strength of an edge between two nodes is calculated using a correlation coefficient computed from a k-nearest-neighbor-based estimator of mutual information68. From these correlations, optimal paths were computed using the Floyd–Warshall algorithm implemented in dynetan (1.0.1) and NetworkX69. To illustrate long-range interaction paths, a source node was selected as the first simulated residue of FtsQ (FtsQN20) and target nodes were selected as the catalytic residues of FtsW (FtsWD297) and FtsI (FtsIS307), resulting in two ensembles of optimal paths.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors thank all members of the Hensel, Xiao, and Lau laboratories for helpful discussions and feedback on the manuscript. Z.H. received support for this work from FCT—Fundação para a Ciência e a Tecnologia, I.P., through MOSTMICRO-ITQB R&D Unit (UIDB/04612/2020, UIDP/04612/2020, Z.H.) and LS4FUTURE Associated Laboratory (LA/P/0087/2020, Z.H.), from a joint research agreement with the Okinawa Institute of Science and Technology, and from the Google Cloud Research Credits program with the award GCP20210916. S.F.C. received support from FCT Fundação para a Ciência e a Tecnologia, I.P., through a PhD fellowship (PD/BD/135480/2018, SMC). Work in the Xiao laboratory was supported by NIH F32GM143895 (B.M.B.), NIH T32GM007445 (J.W.M), and R35GM136436 (J.X.). Work in the Lau laboratory was funded by the Johns Hopkins Catalyst Award (to A.Y.L.); NIH T32GM135131 (to R.A.Y.). Anton 2 computer time (MCB130045P) was provided by the Pittsburgh Supercomputing Center (PSC) through NIH grant R01GM116961 (to A.Y.L.); the Anton 2 machine at PSC was generously made available by D.E. Shaw Research. We also used resources provided by Advanced Research Computing at Hopkins (ARCH) at Johns Hopkins University.
Source data
Author contributions
B.M.B., J.X., and Z.H. conceived the experiments. R.A.Y. and A.Y.L. designed the simulation workflow. B.M.B. and J.W.M. constructed plasmids and strains for imaging experiments. B.M.B. performed single molecule and phenotype imaging, genetic experiments, and all imaging analysis. R.A.Y. performed molecular dynamic simulations. R.A.Y. and Z.H. wrote analysis code. B.M.B., R.A.Y., A.Y.L, J.X., and Z.H. analyzed molecular dynamics data. S.C. and Z.H. analyzed sequence conservation. B.M.B., R.A.Y., J.X., and Z.H. wrote the original draft. All authors reviewed and edited the manuscript. B.M.B., J.W.M., J.X., and Z.H. acquired funding.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
Source data are provided with this paper as a Source Data file. The all-atom trajectories data generated in this study have been made available in the Anton 2 database [https://antonweb.psc.edu/trajectories/]. Trajectories of protein atoms and analysis code have been deposited in the Zenodo database under accession code 10.5281/zenodo.8042906 [https://zenodo.org/record/8042906]. The hydrogen-bonding data has been provided as Supplementary Data 1. Initial model coordinates and coordinates of each system after 1 μs of simulation are included as Supplementary Data 2. PDB Data: 5OIZ, 7ONO, 5Z2W, 6PL5, 8BH1. Source data are provided with this paper.
Code availability
Code for analyzing single-molecule tracking data is available from the Xiao Laboratory GitHub repository (XiaoLabJHU; https://github.com/XiaoLabJHU/SMT_Unwrapping)17. The code for analyzing the MD data have been deposited in the Zenodo database under accession code 10.5281/zenodo.8042906 [https://zenodo.org/record/8042906].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Brooke M. Britton, Remy A. Yovanno.
Contributor Information
Jie Xiao, Email: xiao@jhmi.edu.
Zach Hensel, Email: zach.hensel@itqb.unl.pt.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-39921-4.
References
- 1.Schneider T, Sahl HG. An oldie but a goodie - cell wall biosynthesis as antibiotic target pathway. Int. J. Med. Microbiol. 2010;300:161–169. doi: 10.1016/j.ijmm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Silver LL. Does the cell wall of bacteria remain a viable source of targets for novel antibiotics? Biochem Pharmacol. 2006;71:996–1005. doi: 10.1016/j.bcp.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Paulussen FM, et al. Covalent proteomimetic inhibitor of the bacterial FtsQB divisome complex. J. Am. Chem. Soc. 2022;144:15303–15313. doi: 10.1021/jacs.2c06304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi EF, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 5.Nanninga N. Cell division and peptidoglycan assembly in Escherichia coli. Mol. Microbiol. 1991;5:791–795. doi: 10.1111/j.1365-2958.1991.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 6.Levin PA, Janakiraman A. Localization, assembly, and activation of the Escherichia coli cell division machinery. EcoSal. 2021;9:eESP00222021. doi: 10.1128/ecosalplus.ESP-0022-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taguchi A, et al. FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin-binding protein. Nat. Microbiol. 2019;4:587–594. doi: 10.1038/s41564-018-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraipont C, et al. The integral membrane FtsW protein and peptidoglycan synthase PBP3 form a subcomplex in Escherichia coli. Microbiology. 2011;157:251–259. doi: 10.1099/mic.0.040071-0. [DOI] [PubMed] [Google Scholar]
- 9.Marmont LS, Bernhardt TG. A conserved subcomplex within the bacterial cytokinetic ring activates cell wall synthesis by the FtsW-FtsI synthase. Proc. Natl. Acad. Sci. USA. 2020;117:23879–23885. doi: 10.1073/pnas.2004598117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park, K. T., Du, S. & Lutkenhaus, J. Essential role for FtsL in activation of septal peptidoglycan synthesis. mBio1110.1128/mBio.03012-20 (2020). [DOI] [PMC free article] [PubMed]
- 11.Tsang MJ, Bernhardt TG. A role for the FtsQLB complex in cytokinetic ring activation revealed by an ftsL allele that accelerates division. Mol. Microbiol. 2015;95:925–944. doi: 10.1111/mmi.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du S, Lutkenhaus J. Assembly and activation of the Escherichia coli divisome. Mol. Microbiol. 2017;105:177–187. doi: 10.1111/mmi.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerding MA, et al. Self-enhanced accumulation of FtsN at division sites and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. J. Bacteriol. 2009;191:7383–7401. doi: 10.1128/JB.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yahashiri A, Jorgenson MA, Weiss DS. Bacterial SPOR domains are recruited to septal peptidoglycan by binding to glycan strands that lack stem peptides. Proc. Natl. Acad. Sci. USA. 2015;112:11347–11352. doi: 10.1073/pnas.1508536112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Persons L, Lee L, de Boer PA. Roles for both FtsA and the FtsBLQ subcomplex in FtsN-stimulated cell constriction in Escherichia coli. Mol. Microbiol. 2015;95:945–970. doi: 10.1111/mmi.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichoff S, Du S, Lutkenhaus J. Disruption of divisome assembly rescued by FtsN-FtsA interaction in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2018;115:E6855–E6862. doi: 10.1073/pnas.1806450115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, et al. A two-track model for the spatiotemporal coordination of bacterial septal cell wall synthesis revealed by single-molecule imaging of FtsW. Nat. Microbiol. 2021;6:584–593. doi: 10.1038/s41564-020-00853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyu Z, et al. FtsN maintains active septal cell wall synthesis by forming a processive complex with the septum-specific peptidoglycan synthases in E. coli. Nat. Commun. 2022;13:5751. doi: 10.1038/s41467-022-33404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boes, A., Olatunji, S., Breukink, E. & Terrak, M. Regulation of the peptidoglycan polymerase activity of PBP1b by antagonist actions of the core divisome proteins FtsBLQ and FtsN. mBio1010.1128/mBio.01912-18 (2019). [DOI] [PMC free article] [PubMed]
- 20.Li Y, et al. Genetic analysis of the septal peptidoglycan synthase FtsWI complex supports a conserved activation mechanism for SEDS-bPBP complexes. PLoS Genet. 2021;17:e1009366. doi: 10.1371/journal.pgen.1009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wissel MC, Weiss DS. Genetic analysis of the cell division protein FtsI (PBP3): amino acid substitutions that impair septal localization of FtsI and recruitment of FtsN. J. Bacteriol. 2004;186:490–502. doi: 10.1128/JB.186.2.490-502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attaibi, M. & den Blaauwen, T. An updated model of the divisome: regulation of the septal peptidoglycan synthesis machinery by the divisome. Int. J. Mol. Sci.2310.3390/ijms23073537(2022). [DOI] [PMC free article] [PubMed]
- 23.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craven SJ, Condon SGF, Diaz Vazquez G, Cui Q, Senes A. The coiled-coil domain of Escherichia coli FtsLB is a structurally detuned element critical for modulating its activation in bacterial cell division. J. Biol. Chem. 2022;298:101460. doi: 10.1016/j.jbc.2021.101460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv10.1101/2021.10.04.463034 (2022).
- 26.Baek M, et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science. 2021;373:871–876. doi: 10.1126/science.abj8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryant P, Pozzati G, Elofsson A. Improved prediction of protein-protein interactions using AlphaFold2. Nat. Commun. 2022;13:1265. doi: 10.1038/s41467-022-28865-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modi V, Dunbrack RL. Kincore: a web resource for structural classification of protein kinases and their inhibitors. Nucleic Acids Res. 2022;50:D654–D664. doi: 10.1093/nar/gkab920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pak, M. A. et al. Using AlphaFold to predict the impact of single mutations on protein stability and function. PLoS One18, e0282689 (2023). [DOI] [PMC free article] [PubMed]
- 30.McCausland JW, et al. Treadmilling FtsZ polymers drive the directional movement of sPG-synthesis enzymes via a Brownian ratchet mechanism. Nat. Commun. 2021;12:609. doi: 10.1038/s41467-020-20873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, et al. GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science. 2017;355:744–747. doi: 10.1126/science.aak9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buddelmeijer N, Beckwith J. A complex of the Escherichia coli cell division proteins FtsL, FtsB and FtsQ forms independently of its localization to the septal region. Mol. Microbiol. 2004;52:1315–1327. doi: 10.1111/j.1365-2958.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 33.Mirdita M, et al. ColabFold: making protein folding accessible to all. Nat. Methods. 2022;19:679–682. doi: 10.1038/s41592-022-01488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu S, Wallner B. DockQ: a quality measure for protein-protein docking models. PLoS ONE. 2016;11:e0161879. doi: 10.1371/journal.pone.0161879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales Angeles D, Macia-Valero A, Bohorquez LC, Scheffers DJ. The PASTA domains of Bacillus subtilis PBP2B strengthen the interaction of PBP2B with DivIB. Microbiology. 2020;166:826–836. doi: 10.1099/mic.0.000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernardo-García N, et al. Allostery, recognition of nascent peptidoglycan, and cross-linking of the cell wall by the essential penicillin-binding protein 2x of Streptococcus pneumoniae. ACS Chem. Biol. 2018;13:694–702. doi: 10.1021/acschembio.7b00817. [DOI] [PubMed] [Google Scholar]
- 37.Robichon C, Karimova G, Beckwith J, Ladant D. Role of leucine zipper motifs in association of the Escherichia coli cell division proteins FtsL and FtsB. J. Bacteriol. 2011;193:4988–4992. doi: 10.1128/JB.00324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sjodt M, et al. Structure of the peptidoglycan polymerase RodA resolved by evolutionary coupling analysis. Nature. 2018;556:118–121. doi: 10.1038/nature25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjodt M, et al. Structural coordination of polymerization and crosslinking by a SEDS-bPBP peptidoglycan synthase complex. Nat. Microbiol. 2020;5:813–820. doi: 10.1038/s41564-020-0687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freischem, S. et al. Interaction Mode of the Novel Monobactam AIC499 Targeting Penicillin Binding Protein 3 of Gram-Negative Bacteria. Biomolecules 2021, 11, 1057. Biomolecules12 (2021). [DOI] [PMC free article] [PubMed]
- 41.Kureisaite-Ciziene, D. et al. Structural analysis of the interaction between the bacterial cell division proteins FtsQ and FtsB. mBio910.1128/mBio.01346-18 (2018). [DOI] [PMC free article] [PubMed]
- 42.Kashammer L, et al. Cryo-EM structure of the bacterial divisome core complex and antibiotic target FtsWIQBL. Nat. Microbiol. 2023;8:1149–1159. doi: 10.1038/s41564-023-01368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, et al. Identification of the potential active site of the septal peptidoglycan polymerase FtsW. PLoS Genet. 2022;18:e1009993. doi: 10.1371/journal.pgen.1009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park, K. T., Pichoff, S., Du, S. & Lutkenhaus, J. FtsA acts through FtsW to promote cell wall synthesis during cell division in Escherichia coli. Proc. Natl. Acad. Sci. USA11810.1073/pnas.2107210118 (2021). [DOI] [PMC free article] [PubMed]
- 45.Choi Y, et al. Structural insights into the FtsQ/FtsB/FtsL complex, a key component of the divisome. Sci. Rep. 2018;8:18061. doi: 10.1038/s41598-018-36001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craven, S. J., Condon, S. G. F. & Senes, A. A model of the interactions between the FtsQLB and the FtsWI peptidoglycan synthase complex in bacterial cell division. Preprint at bioRxiv10.1101/2022.10.30.514410 (2022).
- 47.Nagasawa H, et al. Determination of the cleavage site involved in C-terminal processing of penicillin-binding protein 3 of Escherichia coli. J. Bacteriol. 1989;171:5890–5893. doi: 10.1128/jb.171.11.5890-5893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hara H, et al. Genetic analyses of processing involving C-terminal cleavage in penicillin-binding protein 3 of Escherichia coli. J. Bacteriol. 1989;171:5882–5889. doi: 10.1128/jb.171.11.5882-5889.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez MD, Akbay EA, Boyd D, Beckwith J. Multiple interaction domains in FtsL, a protein component of the widely conserved bacterial FtsLBQ cell division complex. J. Bacteriol. 2010;192:2757–2768. doi: 10.1128/JB.01609-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goehring NW, Petrovska I, Boyd D, Beckwith J. Mutants, suppressors, and wrinkled colonies: mutant alleles of the cell division gene ftsQ point to functional domains in FtsQ and a role for domain 1C of FtsA in divisome assembly. J. Bacteriol. 2007;189:633–645. doi: 10.1128/JB.00991-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Condon SGF, et al. The FtsLB subcomplex of the bacterial divisome is a tetramer with an uninterrupted FtsL helix linking the transmembrane and periplasmic regions. J. Biol. Chem. 2018;293:1623–1641. doi: 10.1074/jbc.RA117.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park, K. T., Du, S. & Lutkenhaus, J. Essential Role for FtsL in Activation of Septal Peptidoglycan Synthesis. mBio11, 10.1128/mBio.03012-20 (2020). [DOI] [PMC free article] [PubMed]
- 53.Brown AM, Zondlo NJ. A propensity scale for type II polyproline helices (PPII): aromatic amino acids in proline-rich sequences strongly disfavor PPII due to proline-aromatic interactions. Biochemistry. 2012;51:5041–5051. doi: 10.1021/bi3002924. [DOI] [PubMed] [Google Scholar]
- 54.Williams KP, et al. Phylogeny of gammaproteobacteria. J. Bacteriol. 2010;192:2305–2314. doi: 10.1128/JB.01480-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao B, Mohan R, Gupta RS. Phylogenomics and protein signatures elucidating the evolutionary relationships among the Gammaproteobacteria. Int. J. Syst. Evol. Microbiol. 2009;59:234–247. doi: 10.1099/ijs.0.002741-0. [DOI] [PubMed] [Google Scholar]
- 56.Melo MCR, Bernardi RC, Fuente-Nunez CDI, Luthey-Schulten Z. Generalized correlation-based dynamical network analysis: a new high-performance approach for identifying allosteric communications in molecular dynamics trajectories. J. Chem. Phys. 2020;153:134104. doi: 10.1063/5.0018980. [DOI] [PubMed] [Google Scholar]
- 57.Gómez, M. J., Desviat, L. R., Merchante, R. & Ayala, J. A. in Bacterial Growth and Lysis: Metabolism and Structure of the Bacterial Sacculus (eds de Pedro, M. A. et al.) 309–318 (Springer US, 1993).
- 58.Ovesný M, Křížek P, Borkovec J, Svindrych Z, Hagen GM. ThunderSTORM: a comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging. Bioinformatics. 2014;30:2389–2390. doi: 10.1093/bioinformatics/btu202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinegger M, Soding J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 2017;35:1026–1028. doi: 10.1038/nbt.3988. [DOI] [PubMed] [Google Scholar]
- 60.Nierhaus T, et al. Bacterial divisome protein FtsA forms curved antiparallel double filaments when binding to FtsN. Nat. Microbiol. 2022;7:1686–1701. doi: 10.1038/s41564-022-01206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendler K, et al. AnnoTree: visualization and exploration of a functionally annotated microbial tree of life. Nucleic Acids Res. 2019;47:4442–4448. doi: 10.1093/nar/gkz246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926–935. doi: 10.1063/1.445869. [DOI] [Google Scholar]
- 65.Kräutler V, van Gunsteren WF, Hünenberger PH. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001;22:501–508. doi: 10.1002/1096-987X(20010415)22:5<501::AID-JCC1021>3.0.CO;2-V. [DOI] [Google Scholar]
- 66.Tuckerman M, Berne BJ, Martyna GJ. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 1992;97:1990–2001. doi: 10.1063/1.463137. [DOI] [Google Scholar]
- 67.Shan Y, Klepeis JL, Eastwood MP, Dror RO, Shaw DE. Gaussian split Ewald: a fast Ewald mesh method for molecular simulation. J. Chem. Phys. 2005;122:054101. doi: 10.1063/1.1839571. [DOI] [PubMed] [Google Scholar]
- 68.Melo MCR, Bernardi RC, de la Fuente-Nunez C, Luthey-Schulten Z. Generalized correlation-based dynamical network analysis: a new high-performance approach for identifying allosteric communications in molecular dynamics trajectories. J. Chem. Phys. 2020;153:134104. doi: 10.1063/5.0018980. [DOI] [PubMed] [Google Scholar]
- 69.Hagberg, A., Swart, P. & Chult, D. Exploring Network Structure, Dynamics, and Function Using NetworkX. Proceedings of the 7th Python in Science Conference. (2008).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Source data are provided with this paper as a Source Data file. The all-atom trajectories data generated in this study have been made available in the Anton 2 database [https://antonweb.psc.edu/trajectories/]. Trajectories of protein atoms and analysis code have been deposited in the Zenodo database under accession code 10.5281/zenodo.8042906 [https://zenodo.org/record/8042906]. The hydrogen-bonding data has been provided as Supplementary Data 1. Initial model coordinates and coordinates of each system after 1 μs of simulation are included as Supplementary Data 2. PDB Data: 5OIZ, 7ONO, 5Z2W, 6PL5, 8BH1. Source data are provided with this paper.
Code for analyzing single-molecule tracking data is available from the Xiao Laboratory GitHub repository (XiaoLabJHU; https://github.com/XiaoLabJHU/SMT_Unwrapping)17. The code for analyzing the MD data have been deposited in the Zenodo database under accession code 10.5281/zenodo.8042906 [https://zenodo.org/record/8042906].