FIGURE 4.
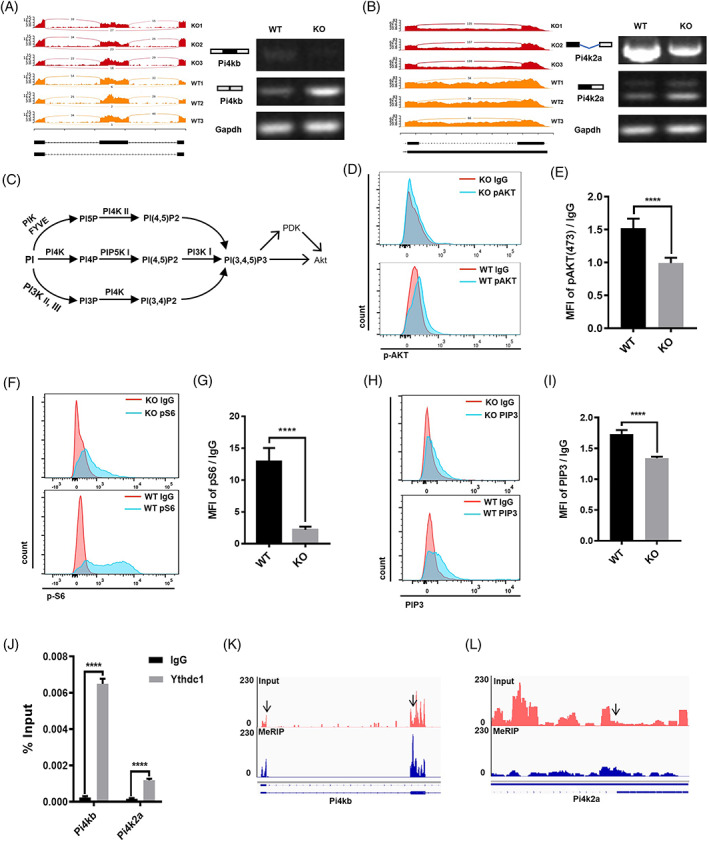
Ythdc1‐null satellite cells (SCs) show deficiencies in PI4K–Akt–mTOR signalling. (A,B) Gene track view and reverse‐transcription (RT)‐PCR validation of exon skipping in Pi4kb (A) and intron retention in Pi4k2a (B). (C) Phosphatidylinositol metabolism and Akt. (D) Phosphorylated Akt‐473 in SCs by Day 2.5 post‐injury were determined by fluorescence‐activated cell sorting (FACS). (E) Quantification of phosphorylated Akt‐473 showed in (D; wild type [WT], n = 5; knockout [KO], n = 5). (F) pS6 protein in SCs by Day 2.5 post‐injury were determined by FACS. (G) Quantification of pS6 protein showed in (F; WT, n = 5; O, n = 5). (H) PI 3,4,5‐trisphosphate (PIP3) content in SCs by Day 30 post‐TMX administration was determined by FACS. (I) Quantification of PIP3 content showed in (H; WT, n = 4; KO, n = 4). (J) RNA immunoprecipitation‐RT‐qPCR assay with antibody against Ythdc1 was performed in C2C12 myoblast (n = 3). (K,L) IGV tracks displaying N6‐methyladenosine‐enriched Pi4kb (K) and Pi4k2a (L) in C2C12 myoblast. Data represent mean ± SD. Statistical analysis was performed using unpaired two‐tailed Student's t‐test (****p < 0.0001).
