Introduction
The pharmacokinetic (PK) variability of infliximab (anti-TNF) in children with inflammatory bowel disease (IBD) likely contributes to the inconsistent rates of clinical remission and endoscopic healing observed with standard (as-labeled) dosing.1 Although proactive or reactive therapeutic drug monitoring (TDM) will alert clinicians of antitumor necrosis factor (anti-TNF) concentrations below target, early immunogenicity is common in children2,3; and empiric (“trial and error”) dose intensifications may delay the time to achieve the targeted concentration.4 Moreover, studies have consistently shown that higher drug exposure during induction is associated with superior rates of early remission.2,5 Interestingly, it was recently shown that a strategy of using model-informed precision dose (MIPD) with a PK dashboard was associated with a reduction in the loss of response during infliximab maintenance in adults with IBD.6
Given the limited therapeutic options for children and the high rates of immunogenicity to the anti-TNF biologics,2,3 our multidisciplinary team identified a critical unmet need for a PK dashboard capable of bedside MIPD to more rapidly (during induction) and accurately attain targeted concentrations. In this study, we highlight the formal process to create and integrate the RoadMAB dashboard within the electronic health record (EHR) and the results of using MIPD for infliximab at our center.
Methods
The dashboard development team included an MIPD software consultant (N.P.), clinical pharmacologists (A.A.V., T.M.), pediatric gastroenterologists (P.M. and others acknowledged), software architects, and a human factors engineering firm (POMIET, Dayton, OH, USA). During the early development stage, the team recognized there was a need for an intuitive, interactive, and easy to access dashboard that would launch within the EHR (Epic, Verona, WI) and a standalone dashboard (web portal, available online) capable of performing MIPD for both induction and maintenance therapy.
Technical Dashboard Development
The team first created the infliximab “demonstrator” dashboard (image not shown) as both a basic proof of concept PK dashboard and a template for our future MIPD platform. In order to refine the demonstrator for use by clinicians and operate within the EHR, the team outlined a design sprint conducted over 4 distinct phases with structured interviews to identify the requirements of users (physicians and advanced practitioners) and create design wireframes (prototypes) for the infliximab dashboard. Phase 1 included interviews with clinical staff (nurses, physicians, and clinical pharmacologists) to review the existing workflow and processes for infliximab dose selection at the start of and during therapy to identify potential weaknesses with using the current EHR system to dose optimize infliximab. In phase 2, the team analyzed participant’s responses, identified decision support needs, and created low fidelity dashboard design wireframes for initial physician review. By the end of phase 3, the redesigned dashboard was again presented to physicians for feedback, and additional modifications (page layout, responder “must-haves,” and dashboard advanced features) were made based on consensus. During design phase 4, physicians were presented with restructured wireframes with higher fidelity and an interactive (clickable) prototype using the InVision (InVisionApp Inc.) platform, which allows users to trial and edit the design interface prior to final production.
From a technical aspect, the dashboard coding for the backend (server-side) is written in C#.NET and is primarily constructed with an application programming interface (API) that allows the PK dashboard (frontend display) to launch within the local EHR (Epic). In order for the dashboard to run smoothly, it was key to extract patient data (medication administration history and laboratory results) in real time with the use of Fast Healthcare Interoperability Resources (FHIR) technology.
The final PK dashboard is equipped with 2 primary displays, the induction display (“New Start Wizard”) that opens from infusions 1-3 and the maintenance display that opens starting at infusion 4 and all subsequent infusions. Our team also created the RoadMAB portal (portal.roadmab.org) as a standalone replica of the EHR dashboard that has the same functionality; however, it requires manual entry of patient data and is only available by accessing a secured website.
The System Usability Scale7 (SUS) was used during structured interviews to objectively assess the serviceability and usability of the prior and the newly created decision support tools, which included the (1) the current EHR at Cincinnati Children’s, (2) RoadMAB Epic version 1, and (3) RoadMAB Epic version 5.
Results
Eleven health care professionals (physicians, nurses, and clinical pharmacologists) participated in the design sprints over 26 sessions. The SUS was completed after 3 distinct time points by 10 separate physicians with varied clinical experience (mean 18 years, standard deviation [SD] 14) and number of IBD patients seen per month (mean 25 patients, SD 23).
During phase 1, the mean (SD) SUS for the current process (using Epic without a PK dashboard) for infliximab dose optimizations was 63.1 of 100 (SD 14.8, n = 4 physicians). Following user feedback, stepwise modifications were made to the wireframes (up to phase 4) for RoadMAB. The mean SUS for RoadMAB EHR, version 1 was 76 of 100 (SD 14.3, n = 4 physicians). Following a series of additional redesign sessions, the mean SUS for RoadMAB EHR, version 5 was 86.5 of 100 (SD 15.2, n = 5 physicians).
RoadMAB Features
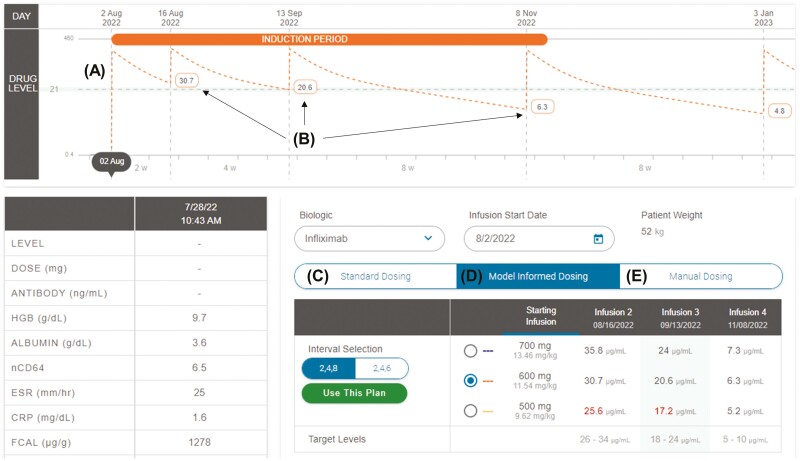
We created the New Start Wizard (Figure 1) within RoadMAB to simplify MIPD for infliximab induction by quickly incorporating baseline laboratory results (covariates of drug clearance), conducting Bayesian PK estimation using the selected population PK model, and displaying multiple projected concentration-time profiles. During maintenance therapy, RoadMAB also rapidly incorporates past drug administrations and laboratory results (including TDM) to display multiple projected concentration-time profiles and the corresponding (predicted) trough concentrations for 2 subsequent infusions. Advanced dashboard features include user modification of the default targeted trough concentration range during induction and maintenance, selection of up to five different population PK models, and electing the specific TDM assay. The same user interface has been optimized for vedolizumab, as well (not shown).
Figure 1.
The RoadMAB New Start Wizard was created to simplify induction dose selection. Prior to receiving the first dose, the New Start Wizard creates a projected (A) concentration-time curve along with (B) future (projected) trough concentrations (at dose 2, dose 3, and dose 4) based on the selected interval and dose. The user can toggle through (C) standard dosing, (D) model-informed dosing, and (E) manual dosing to guide the dosing decision.
TDM Consultations
Prior to the integration of RoadMAB within the EHR, individualized MIPD at our center was available to clinicians as a formal PK consult conducted by the clinical pharmacology pharmacometrics service. During these consults, the clinical pharmacologists (A.A.V., T.M.) provided dose recommendations following TDM using the RoadMAB demonstrator along with Bayesian dosing software MwPharm++ (Medimatics, Maastricht, the Netherlands). Following institutional review board approval, we performed a retrospective review of all infliximab PK consults requested by the primary gastroenterologist from March 2019 to November 2021. In total, we identified 21 IBD patients who had a PK consult during induction (n = 2) and maintenance (n = 19). The median age was 15 years (interquartile range, 11-18) and 76.2% (16 of 21) had Crohn’s disease (Table 1).
Table 1.
Patient characteristics at the time of the pharmacokinetic consult
| Characteristic | n = 21 |
|---|---|
| Age, years; median (IQR) | 15 (11-18) |
| IBD diagnosis, Crohn’s disease; n | 16 (76%) |
| Therapy phase; n in maintenance | 19 (90.4%) |
| Weight-based dose, mg/kg; median (IQR) | 9.7 (7.9-11.2) |
| Interval, weeks; median (IQR) | 4 (4-6) |
| Maintenance trough, µg/mL; median (IQR) | 11 (5.1-23.5) |
| No. with antibodies to infliximab (ATI); n | 6 (28.6%) |
| ATI concentration, ng/mL; median (IQR) | 187 (102-270) |
| Albumin, g/dL; median (IQR) | 3.9 (3.6-4.1) |
| Paris classification; n | |
| Crohn’s location: L1, L2, L3 | 6 (37.5%), 4 (25%), 6 (37.5%) |
| Crohn’s behavior: B1, B2, B3 | 12 (75%), 1 (6.3%), 3 (18.7%) |
| Ulcerative colitis location, E4 | 5 (100%) |
IQR, 25%-75% interquartile range.
Pharmacokinetic consults varied from requests for an increase (n = 10) or decrease (n = 8) in maintenance trough concentrations, requests to forecast an induction regimen (n = 2), and a request to forecast trough concentrations after a bowel resection (n = 1). We found most (12 of 21) MIPD recommendations resulted in (agreed upon) changes in both dose (mg) and interval (weeks) by the treating physician. Partial agreement (dose only or interval only) occurred in 7 of 21 of the consults, with only 2 PK recommendations (both for induction doses) not instituted by the physician. Among the 19 consults during the maintenance phase, a postconsult trough concentration was available within 6 months after the initial consult for all 19 patients. Eighty-four percent (n = 16) of postconsult concentrations were within or above the requested range, and 58% (n = 11) were within the requested narrow concentration range (± 2.5 µg/mL). Among the 3 patients with concentrations below the targeted level, one patient exhibited abnormally high drug clearance despite 16 mg/kg every 4 weeks and ultimately required an ileocecal resection, whereas the second patient had incomplete data (received external infusions) to confirm adherence to the PK recommendations. The third patient required infliximab discontinuation with elevated antibodies to infliximab (ATI) and an undetectable drug concentration secondary to nonadherence. We found when the full recommendation was instituted by the physician, 92% (11 of 12) of the postconsult concentrations were within or above the requested range, and 75% (9 of 12) were within the requested narrow concentration range (± 2.5 µg/mL). Lastly, 5 other patients had ATI at the time of the PK consult. Following the consult, repeat TDM showed 2 had an undetectable ATI, and the other 3 had low level ATI (<250 ng/mL) in the setting of therapeutic concentrations.
Real-world Use of the PK Dashboard
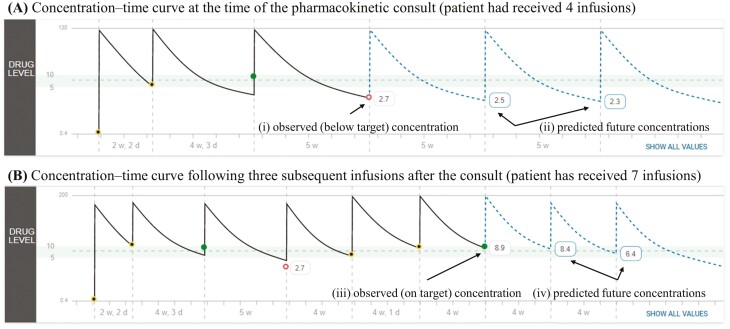
The following PK consult demonstrates the utility of the PK dashboard for a typical patient with rapid drug clearance at the first maintenance dose. Infliximab (10 mg/kg) was started during induction in a 7-year-old child with severe Crohn’s disease. Proactive TDM was performed at the fourth infusion (week 12) and revealed an infliximab trough of 2.7 µg/mL (Figure 2A [i]), and ATI was undetectable (using a drug-tolerant assay). As the trough concentration was below our center’s target (5-10 µg/mL) and subsequent infusions at this dose and frequency (Figure 2A [ii]) were predicted to be below the trough target, a PK consult was performed using a published PK model,8 the patient’s past infliximab administration history, and the available covariates of drug clearance (weight, drug concentration, and albumin). The final recommendation was to increase the dose to 15 mg/kg and escalate the frequency to every 4 weeks. Proactive TDM was repeated prior to the third intensified dose, and the patient’s trough concentration (8.9 µg/mL, Figure 2B [iii]) was within the targeted range, with future trough concentrations predicted to remain within the targeted range (Figure 2B [iv]).
Figure 2.
Real-world use of the maintenance PK dashboard highlighting the utility of model-informed precision dosing for patients found to have rapid drug clearance. The patient’s (A) observed (solid line) concentration-time curve, observed concentration (2.7 µg/mL) and the predicted (dashed-line) future concentrations at the time of the consult. (B) The patient’s observed concentration-time curve and the measured infliximab concentration (8.9 µg/mL) at the third infusion after the consult. The shaded region in each panel represents the targeted trough concentration range (5-10 µg/mL) during infliximab maintenance.
Discussion
With the current technology to perform MIPD at the bedside and the availability of extensive population PK data for infliximab, suboptimal exposure in pediatric IBD should become a rare event. However, low trough concentrations and high rates of immunogenicity are common when standard dosing regimens are used for all patients.2,3,9 Our team has successfully developed the RoadMAB dashboard to improve drug durability, as anti-TNF biologics (infliximab and adalimumab) are the only biologics approved by the United States Food and Drug Administration for children and remain first-line therapy to manage pediatric Crohn’s disease in North America.10
Our dashboard design process led to notable improvements in the SUS from empiric dosing (without a dashboard) to the final design (86.5 of 100) of RoadMAB; as a sidenote, any SUS score >70 is considered above average.7 Additionally, our real-world use of the infliximab dashboard during PK consults demonstrated good precision to achieve individual concentration targets and reduce ATI concentrations for patients receiving maintenance infusions. As a next step, we are currently conducting a clinical trial in children starting infliximab for moderate-severe Crohn’s disease to assess the efficacy and safety of using an innovative infliximab MIPD strategy starting from induction.
It is important to note that the recommendations generated from any PK dashboard, MIPD application, or a PK consult are merely suggestions (tools) to aid treatment decisions, with the clinician ultimately responsible for the final order. In fact, this was evident in our real-world cohort as only 12 of 21 of the MIPD recommendations were instituted. As novel clinical decision support tools are developed to personalize IBD care, it will be vital to further test these innovative technologies in large, prospective clinical trials during all phases of care.
While use of a PK dashboard for precision IBD care is in its infancy, future studies will better define exposure targets for distinct populations (children, patients <30 kg, or perianal Crohn’s). Lastly, it will be key to test whether more favorable outcomes are achieved using a novel treat-to-target precision care (T2T-PC) paradigm that includes the combination of MIPD, proactive TDM and pharmacodynamic monitoring.
Acknowledgments
Authors acknowledge the Information Services Team including Frank Menke and Aaron Vogt, Cincinnati Children’s Hospital Medical Center, Division of Information Services. Authors also thank Kimberly Jackson, Lois Siegle, Renee Etter, Jeffrey Hyams, Kristin Bramlage, Danny Mellon, Emily Evans, Jennifer Hellmann, and Kaitlin Whaley for testing and validating the PK dashboard.
Contributor Information
Ruben J Colman, Division of Gastroenterology, Hepatology and Nutrition, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Abigail Samuels, Department of Medicine, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Tomoyuki Mizuno, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA; Division of Clinical Pharmacology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Nieko Punt, Medimatics, Maastricht, The Netherlands.
Alexander A Vinks, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA; Division of Clinical Pharmacology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Phillip Minar, Division of Gastroenterology, Hepatology and Nutrition, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Author Contribution
Study concept and design: P.M., N.P., A.A.V.
Analysis and interpretation of data; P.M., R.C., A.S., T.M., N.P., A.A.V.
Drafting of the manuscript: P.M., R.C.
Technical support: P.M., A.S., F.M., A.V., N.P., and A.A.V.
Critical revision of the manuscript for important intellectual content: P.M., R.C., A.S., T.M., N.P., A.A.V.
Obtained funding: P.M., A.A.V.
Funding
This work was supported by the National Institutes of Health [K23DK105229 (PM), R03DK118314 (PM), T32DK007727 (RJC)] and the Academic Research Committee from the Cincinnati Children’s Research Foundation (PM, AAV).
Conflicts of Interest
N.P. is president of Medimatics, a company that provides consulting services on medical information systems. All other authors have no financial, professional, or personal arrangement(s) with a company whose product figures prominently in the submitted manuscript or with a company making a competing product.
Data Availability
All data and analytic methods are available upon request to the corresponding author.
References
- 1. D’Arcangelo G, Oliva S, Dilillo A, et al. . Predictors of long-term clinical and endoscopic remission in children with Crohn disease treated with infliximab. J Pediatr Gastroenterol Nutr. 2019;68(6):841–846. [DOI] [PubMed] [Google Scholar]
- 2. Xiong Y, Mizuno T, Colman R, et al. . Real-world infliximab pharmacokinetic study informs an electronic health record-embedded dashboard to guide precision dosing in children with Crohn’s disease. Clin Pharmacol Ther. 2021;109(6):1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kennedy NA, Heap GA, Green HD, et al. ; UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341–353. [DOI] [PubMed] [Google Scholar]
- 4. Vande Casteele N, Ferrante M, Van Assche G, et al. . Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320–9.e3. [DOI] [PubMed] [Google Scholar]
- 5. van Hoeve K, Dreesen E, Hoffman I, et al. . Adequate infliximab exposure during induction predicts remission in paediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2019;68(6):847–853. [DOI] [PubMed] [Google Scholar]
- 6. Strik AS, Lowenberg M, Mould DR, et al. . Efficacy of dashboard driven dosing of infliximab in inflammatory bowel disease patients: a randomized controlled trial. Scand J Gastroenterol. 2021;56(2):145–154. [DOI] [PubMed] [Google Scholar]
- 7. Brooke J. SUS—A quick and dirty usability scale. In: Jordan PW, Thomas B, McClelland IL, Weerdmeester B, eds. Usability Evaluation in Industry. Vol. 189. London: Taylor & Francis; 1996:4–7. [Google Scholar]
- 8. Fasanmade AA, Adedokun OJ, Blank M, Zhou H, Davis HM.. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33(7):946–964. [DOI] [PubMed] [Google Scholar]
- 9. Assa A, Matar M, Turner D, et al. . Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children With Crohn’s disease compared with reactive monitoring. Gastroenterology. 2019;157(4):985–996.e2. [DOI] [PubMed] [Google Scholar]
- 10. Church PC, Hyams J, Ruemmele F, de Ridder L, Turner D, Griffiths AM.. The continental divide: anti-TNF use in pediatric IBD is different in North America compared to other parts of the World. Can J Gastroenterol Hepatol. 2018;2018:3190548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and analytic methods are available upon request to the corresponding author.