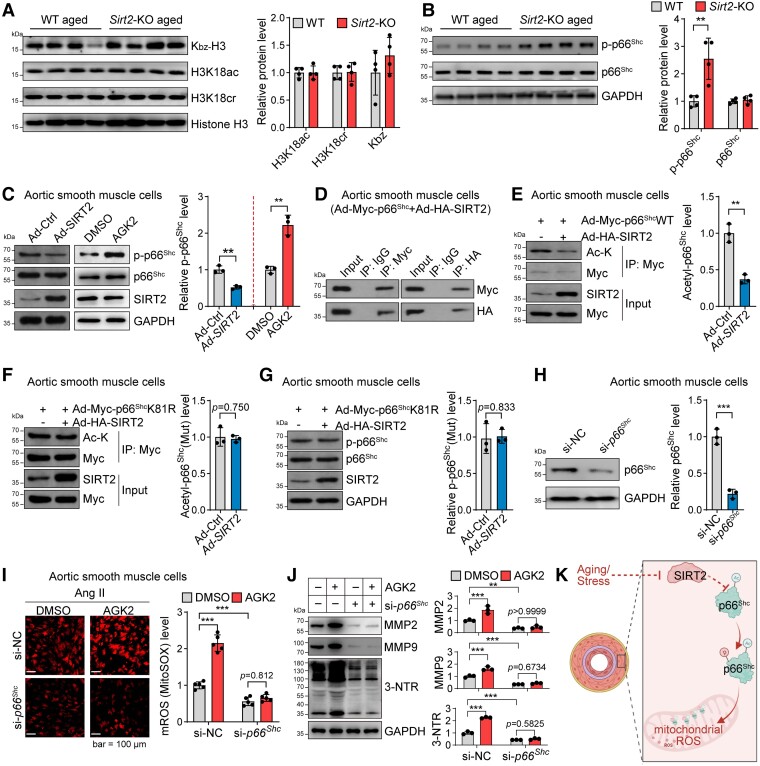
Figure 4.
Sirtuin 2 regulates oxidative stress by deacetylating p66Shc. (A) Sirtuin 2 effects on histone modification in aortas from aged mice. Representative western blot and quantitative results are shown (n = 4). (B) Sirtuin 2 deficiency increased p66Shc phosphorylation in the aortas of aged mice. Representative and quantitative results are shown (n = 4). (C) Sirtuin 2 repressed p66Shc phosphorylation in aortic smooth muscle cells. Aortic smooth muscle cells were infected with an adenovirus carrying sirtuin 2/control adenovirus or treated with the sirtuin 2 inhibitor AGK2 (10 μM)/DMSO for 24 h, followed by western blot analysis. Representative and quantitative results are shown (n = 3). (D) Sirtuin 2 interacted with p66Shc in aortic smooth muscle cells. Aortic smooth muscle cells were infected with an adenovirus carrying Myc-tagged p66Shc and HA-tagged sirtuin 2, followed by immunoprecipitation with Myc and HA antibodies and western blot analysis (n = 3). (E) Sirtuin 2 deacetylated p66Shc in aortic smooth muscle cells. Aortic smooth muscle cells were infected with Ad-Myc-p66Shc with Ad-SIRT2 or Ad-Ctrl, followed by immunoprecipitation with anti-Myc antibodies and western blot analysis of acetylated lysine (Ac-K) on p66Shc (n = 3). (F, G) Sirtuin 2 deacetylated p66Shc at lysine 81 (K81) to inhibit p66Shc phosphorylation in aortic smooth muscle cells. Aortic smooth muscle cells were infected with Myc-tagged K81R (lysine to arginine) mutant p66Shc (Ad-Myc-p66ShcK81R) with Ad-SIRT2 or Ad-Ctrl, followed by immunoprecipitation with anti-Myc antibodies and western blot analysis of acetylated lysine on p66Shc (F) (n = 3) and phosphorylation of p66Shc (G) (n = 3). (H) siRNA-mediated knockdown of p66Shc in aortic smooth muscle cells. The cells were transfected with si-NC or si-p66Shc for 48 h. Then, a western blot was performed to analyse protein expression (n = 3). (I) p66Shc contributed to sirtuin 2 function in regulating mitochondrial reactive oxygen species in aortic smooth muscle cells. Aortic smooth muscle cells were transfected with si-p66Shc or control si-NC for 24 h, followed by treatment with/without the sirtuin 2–specific inhibitor AGK2 (10 μM) and angiotensin II (1 μM) for another 24 h. Mitochondrial reactive oxygen species were monitored by MitoSOX staining. Representative and quantitative results are shown (n = 5). (J) p66Shc contributed to sirtuin 2 function in regulating protein oxidation and matrix metalloproteinase expression in aortic smooth muscle cells. The cells were treated as in (I), and protein expression was analysed by western blot. Representative blots and quantitative results are shown (n = 3). (K) Illustration showing that sirtuin 2 regulates p66Shc acetylation and phosphorylation to repress mitochondrial reactive oxygen species in vascular cells. Statistical analyses were performed using unpaired Student’s t-test (A–H) and one-way ANOVA with the Bonferroni post hoc test (I, J). **P < .01, ***P < .001.