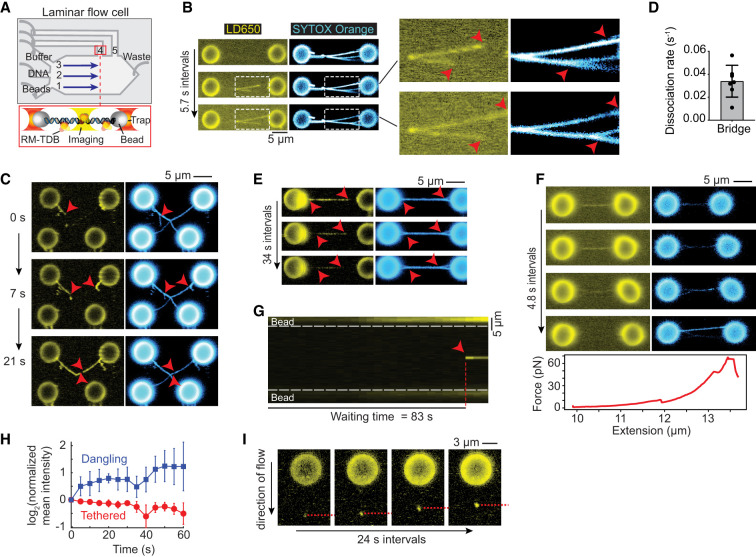
Figure 5.
Single-molecule fluorescence imaging of interactions of the RM-TDB domain with DNA. (A) Schematic of the experimental setup (not to scale). Streptavidin-coated beads, biotinylated λ-DNA, and PBS buffer were separated by laminar flow in channels 1–3, respectively. After tether formation, beads were moved to channel 4 or 5 for protein loading and imaging. The RM-TDB protein concentration in all C-trap experiments was 20 nM. (B) Bridging of multiple tethered DNA duplexes by the RM-TDB domain. Arrowheads in insets indicate where separate DNA molecules branch apart, coinciding with the ends of RM-TDB tracks (see also Supplemental Movie S1). (C) Dangling DNA bundled together with stretched tethers. Arrowheads indicate dangling λ-DNA molecules (i.e., attached to only one bead) that are initially stretched out by flow but become progressively coaligned with segments from the tethers connecting the top pair of beads. Bundling of the dangling DNA with the tethers is coincident with extension of tracks of RM-TDB binding. Note that the single DNA tether that connects the bottom pair of beads did not acquire any coating by the RM-TDB (see also Supplemental Movie S2). (D,E) Quantification of protein dissociation rates (D) and a representative example (E) of disassembly of protein–DNA bridges moved into a protein-free channel. Red arrowheads in E indicate locations where the DNA molecules became separated (see also Supplemental Movie S3). Each point in D is a measurement from a single bridge (N = 7; example in Supplemental Fig. S9B); error bars indicate SD. (F) Force-promoted reversal of the RM-TDB bridge assembly. As beads connected by bridged tethers were pulled apart with increasing force, LD650 fluorescent signal decreased over time, indicating that RM-TDB was undergoing net dissociation despite free protein remaining available in the channel. Segments of the coaligned DNA tethers became separated coincident with loss of protein binding. The corresponding force extension curve is plotted below. (G) Example kymograph of sudden focal binding of the RM-TDB domain (red arrowhead) to a stretched tether. The waiting time is the interval between introduction of the beads to the protein channel and first appearance of the focus. The white dashed lines indicate bead boundaries (see also Supplemental Movie S4). (H) Average change in protein fluorescence intensity over time for focal binding events on stretched tethers (red, N = 4) or on dangling DNA (blue, N = 9). The fluorescence signal at each time point was normalized to the signal in the first frame where binding of RM-TDB was detected (see the Materials and Methods). Error bars indicate SD. (I) Accumulation of RM-TDB pulls dangling DNA against flow. A representative example is shown of RM-TDB binding to the tip of a dangling λ-DNA molecule bound to a single bead and stretched by flow. Over time, the protein-bound tip of the dangling DNA retracted upward toward the bead as indicated by the red dashed line (see also Supplemental Movie S5).