Fig. 3.
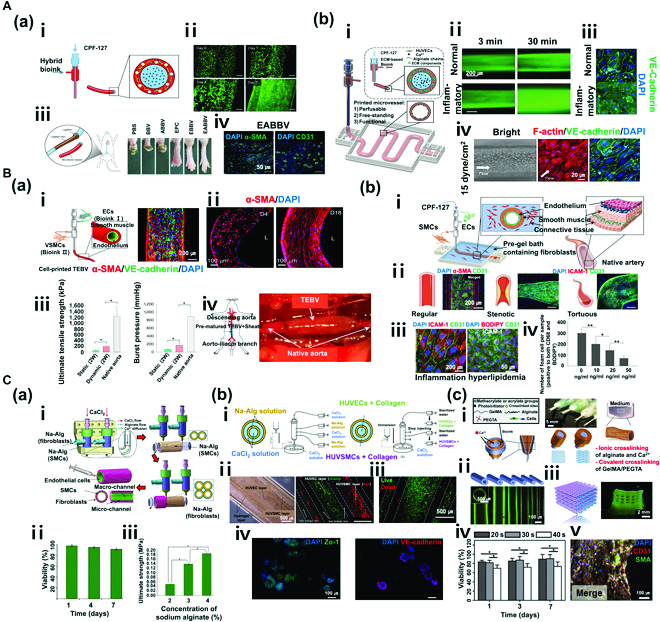
(A) Coaxial nozzle bioprinting (monolayer). (a) Tissue-engineered bio-blood vessels developed using coaxial nozzle cell printing for ischemic disease. (i) Schematic diagram of coaxial nozzle and materials. (ii) Live/dead assay for printed EPC (endothelial progenitor cell)-laden BBV during 7 days. Cells were stained with calcein AM (live, green) and ethidium homodimer I (dead, red). (iii) Representative images of 6 groups (PBS, BBV, ABBV, EPC, EBBV, and EABBV). (iv) Immunostaining results of EPC/APMS-laden BBV. The 6 groups were stained with anti-CD31 antibody and anti-α-SMA antibody after 28 days. BBV, bio-blood vessel; ABBV, atorvastatin-loaded poly (lactic-co-glycolic) acid microsphere (APMS)-laden BBV; EBBV, EPC-laden BBV; EABBV, EPC/APMS-laden BBV. Reproduced with permission [52]. Copyright 2017, The Authors, licensed under a Creative Commons Attribution 4.0 International License. (b) Freestanding, perfusable, and functional in vitro vascular models developed using coaxial nozzle cell printing for mimicking native endothelium pathophysiology. (i) Schematic diagram of coaxial nozzle cell printing process. (ii) Permeability test for a normal vascular model (VM) and an inflammatory model. (iii) Immunostaining results of normal and inflammatory VM indicating disruptions of the endothelial barrier in the inflammatory model. (iv) Response to shear stress in VM. Reproduced with permission [53]. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Coaxial nozzle bioprinting (multilayer). (a) Tissue-engineered vascular grafts containing an endothelial layer and a muscle layer using triple-coaxial cell printing technology. (i) Schematic diagram of triple-coaxial nozzle, materials, and tissue-engineered blood vessels (TEBVs), and immunostaining results of TEBV. (ii) Culturing of TEBV under static conditions (left) and dynamic conditions (right). (iii) Mechanical property test (UTS and BP). (iv) Abdominal aorta graft of TEBV in the rat model. Reproduced with permission [54]. Copyright 2019, AIP Publishing. (b) Fabrication of atherosclerotic in vitro model containing endothelium and smooth muscles. (i) Schematic diagram of the cell printing process, native artery, and materials. (ii) Triple-layer arterial constructs (regular, stenotic, and tortuous). (iii) Endothelial dysfunction response of the arterial construct [inflammation: tumor necrosis factor-α (TNF-α), hyperlipidemia: low-density lipoprotein (LDL)]. (iv) Evaluation of the effect of atorvastatin on an arterial construct (turbulent flow). Reproduced with permission [55]. Copyright 2020, Wiley-VCH GmbH. (C) Vessel-like structure with multilevel fluidic channels. (a) Fabrication process of Na-Alg cell-laden hydrogel vessel-like structure containing endothelium and smooth muscles using coaxial nozzle and rod printing methods. (i) Schematic of the fabrication process. (ii) Live/dead assay (live, green; dead, red) of vessel-like structure. (iii) Test of mechanical properties (UTS) of the construct. Reproduced with permission [56]. Copyright 2017, American Chemical Society. (b) Fabrication of multicellular vessel structure using 4 flow channels. (i) Schematic diagram of the 4-channel coaxial nozzle. (ii) Images showing the distribution of cells via cell-laden hydrogel perfusion. (iii) Live/dead assay (live, green; dead, red). (iv) Immunostaining image showing expression of ZO1 tight junction and VE-cadherin proteins in HUVECs and HUVSMCs. Reproduced with permission [57]. Copyright 2018, Elsevier. (c) Direct 3D bioprinting of perfusable vascular constructs. (i) Schematic diagram of various coaxial nozzle and materials. (ii) 3D-printed vascular constructs with various sizes. (iii) Perfusable 10 layers of a 3D bioprinted construct. (iv) Live/dead assay (live, green; dead, red) of vascular constructs. (v) Immunostaining images showing expression of CD31 and SMA. Reproduced with permission [58]. Copyright 2016, Elsevier.
