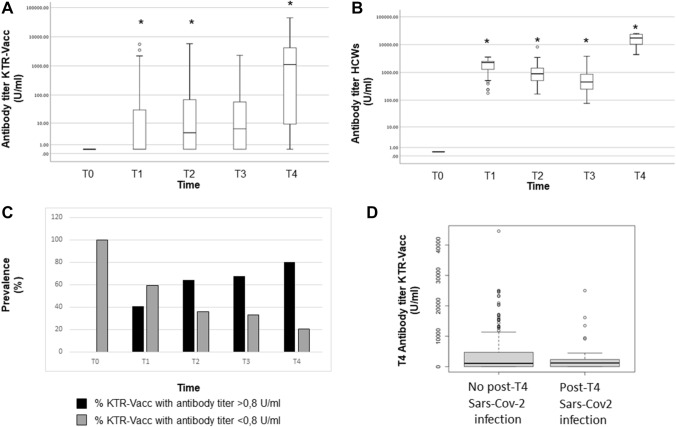
Fig. 1.
Comparison of antibody titers against SARS-CoV-2 Receptor Binding Domain between KTRs (A) and control group (B). Course of anti-SARS-CoV-2 S antibody titers for each patient at baseline (T0), at 14 and 90 days after the second dose (T1 and T2, respectively) and the same day and after 1 month subsequent to the third dose (T3 and T4, respectively). Cut-off for positive test was defined according to the manufacturer’s instructions as a titer ≥ 0.8 U/ml. *p < 0.001 vs previous time. C Prevalence (%) of responders /non responders to BNT162b2 vaccine among KTRs at different time points (T0, T1, T2, T3 and T4). D anti-SARS-CoV-2 S antibody titers at T4 in KTRs who developed COVID-19 infection, and those who did not. p = n.s