Figure 4.
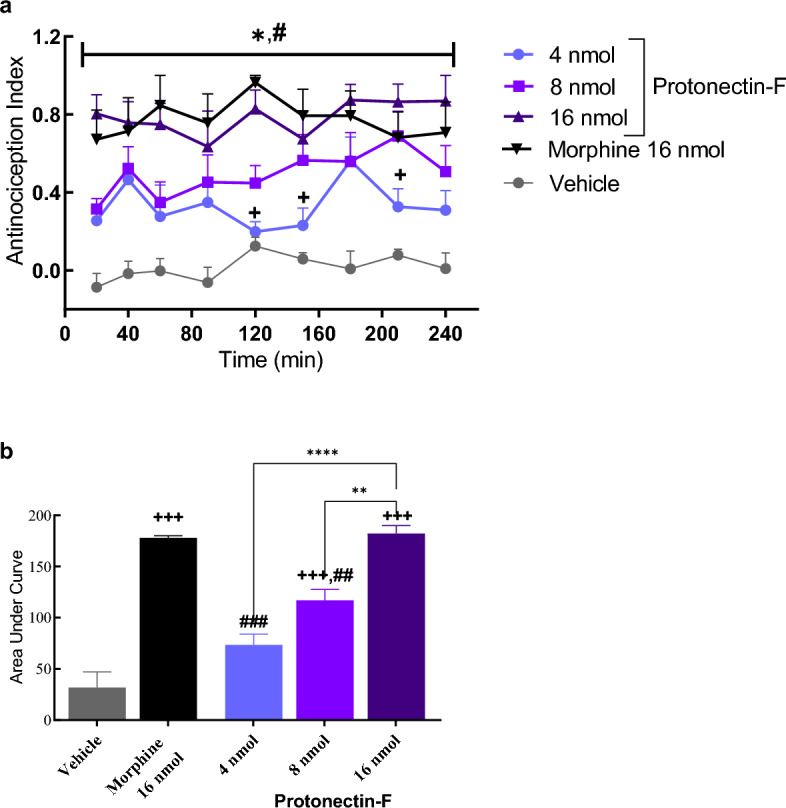
(a) Antinociception index obtained from the hot plate assay after i.c.v. injection of the modified protonectin-F at 16, 8 or 4 nmol/animal. Control groups received either morphine at 16 nmol per animal or vehicle solution. Data were analyzed with Two-Way ANOVA followed by Bonferroni post-hoc test. (*) indicates statistical difference when compared morphine to vehicle control. (#) indicates difference when compared protonectin-F 16 nmol to vehicle control with p < 0.05. ( +) indicates difference when compared to the group treated with protonectin-F at 16 nmol/animal with p < 0.05. (b) Area under curve obtained from the antinociception index results. Data were analyzed by ANOVA followed by Tukey’s post-hoc test. ( +) indicates statistical difference when compared to vehicle control (+++ = p < 0.001). (#) indicates difference when compared to morphine control with p < 0.05 (### = p < 0.001; ## = p < 0.01). (*) indicates difference when compared to the group treated with protonectin-F at 16 nmol/animal (**** = p < 0.0001; ** = p < 0.01).
