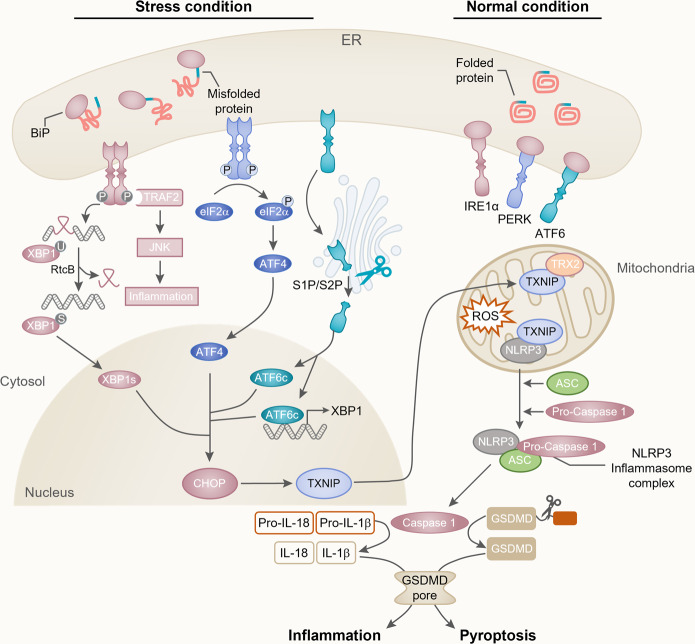
Fig. 2. ER stress-induced CHOP/TXNIP/NLRP3 inflammatory pathway.
The UPR is induced by the accumulation of misfolded proteins in the ER. Disassembly of BiP from IRE1α, PERK, and ATF6 during ER stress activates each domain. Activated IRE1α induces the nonconventional splicing of X-box binding protein-1 (xbp1u; unspliced form) mRNA and forms xbp1s mRNA, which encodes XBP1s (a transcriptional activator). PERK is phosphorylated by ER stress, causing the phosphorylation of eIF2α. This process prevents ribosome assembly, which leads to translational inhibition and allows the cell to deal with ER stress. However, ATF4 dissociates translation inhibition during stress conditions and induces CHOP activation. Under stress conditions, ATF6 is translocated to the Golgi compartment and cleaved by S1P/S2P enzymes. Cleaved ATF6 (ATF6c) induces CHOP and promotes the expression of XBP1s. CHOP induces TXNIP shuttling from the nucleus to the mitochondria, which is required for the generation of mitochondrial ROS. The induction of CHOP/TXNIP signaling activates the NLRP3 inflammasome. Ultimately, active caspase 1 stimulates the maturation of IL-1β/IL-18 and GSDMD cleavage, leading to inflammation and pyroptosis-associated pathways.