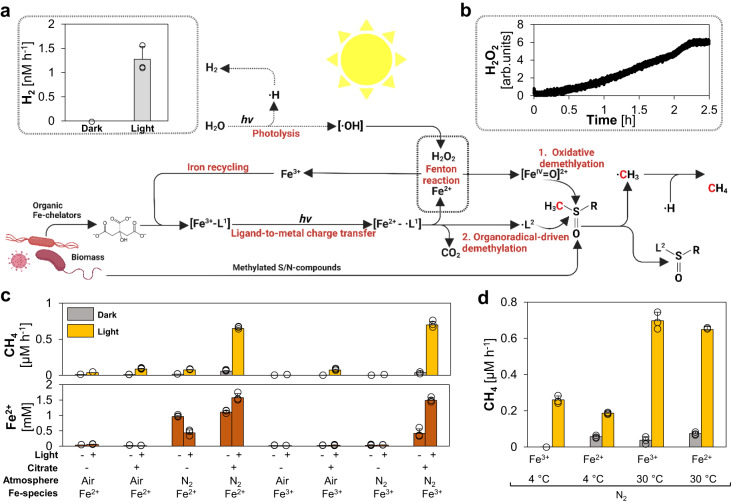
Fig. 3. A light-driven iron redox cycle drives and enhances CH4 formation.
Upon illumination, water is photolytically split into hydroxyl radicals (·OH) and hydrogen forming H2 and H2O2. Organic Fe3+-complexes (Fe3+-[L1]) are converted into Fe2+ and organic radicals (·L1) via ligand-to-metal charge transfer (LMCT). The generated Fe2+ reacts with H2O2 to ·OH or [FeIV= O]2+ and thereby drives the generation of methyl radicals (·CH3) from S-/N-methylated compounds. The LMCT-generated ·L1 decomposes into CO2 and another organic radical (·L2) that additionally facilitates CH4 formation upon reacting with S-/N-methylated compounds. Under light, (a) H2 (gray bars) and (b) H2O2 is formed in pure buffer. c Upon illumination, CH4 formation rates (yellow bars) are increased. Fe2+ formation (brown bars) depends on anoxic conditions and is driven by LMCT induced by the addition of citrate. d Light and heat have synergistic effects on CH4 formation. While heat drives CH4 formation upon Fe2+-supplementation, light increases CH4 formation upon Fe3+- and Fe2+-addition. All experiments were conducted in closed glass vials containing a buffered solution (pH 7) supplemented with DMSO, Fe2+ or Fe3+, N2 or air atmosphere in the presence or absence of citrate incubated under light or in the dark at 4 °C or 30 °C. The bars are the mean + standard deviation of triplicates, shown as circles. a, b Was created with BioRender.com.