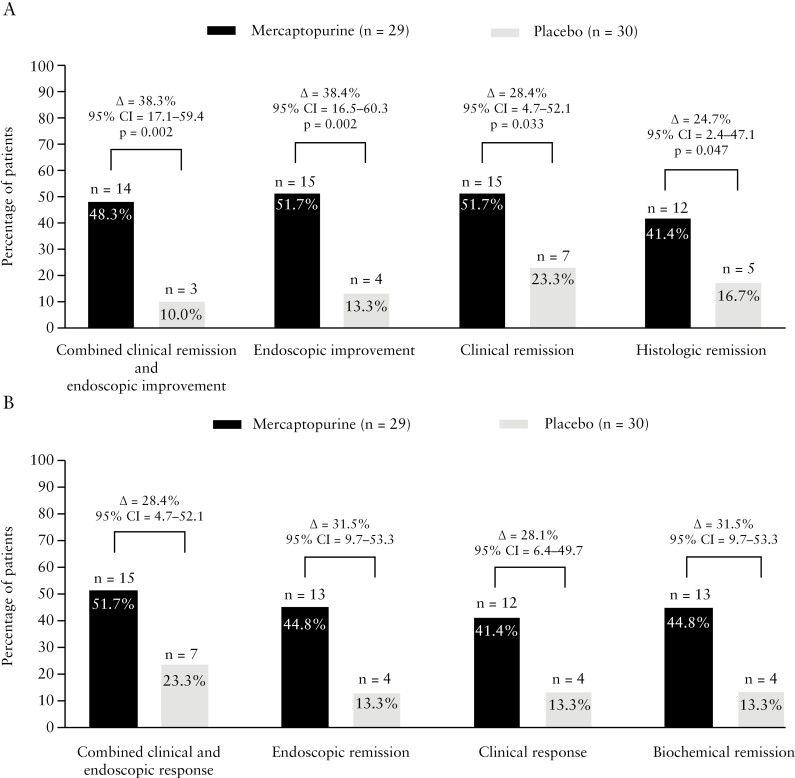
Figure 3.
The proportion of patients achieving corticosteroid-free efficacy endpoints at week 52 in an intention-to-treat analysis. [A] The proportion of patients achieving the primary endpoint: corticosteroid-free combined clinical remission and endoscopic improvement [i.e. total score ≤2, and no item >1, using the 12-point Mayo score consisting of stool frequency, rectal bleeding, endoscopic Mayo score and the physician’s global assessment] and the secondary corticosteroid-free endpoints: endoscopic improvement [i.e. endoscopic Mayo score = 0 or 1], clinical remission [i.e. rectal bleeding score = 0 and stool frequency score = 0 or 1 using the 6-point Mayo score with rectal bleeding and stool frequency score] and histological remission [i.e. absence of neutrophils in the mucosa; Geboes score <2 B.1, Robarts histopathology index ≤3 and/or Nancy score ≤1], at week 52 with delta difference, 95% CI of difference and p-values. [B] The proportion of patients achieving the remaining corticosteroid-free endpoints: combined clinical and endoscopic response [i.e. 3-point and 30% reduction compared to baseline and 1-point drop in the rectal bleeding score or a rectal bleeding score ≤1], endoscopic remission [endoscopic Mayo score = 0], clinical response (≥2-point drop in the 6-point Mayo score [consisting of rectal bleeding and stool frequency items]) compared to baseline and biochemical remission [CRP <5 mg/L and faecal calprotectin <250 mg/kg] at week 52 with the delta percentage difference between groups with 95% confidence intervals.