Fig. 4.
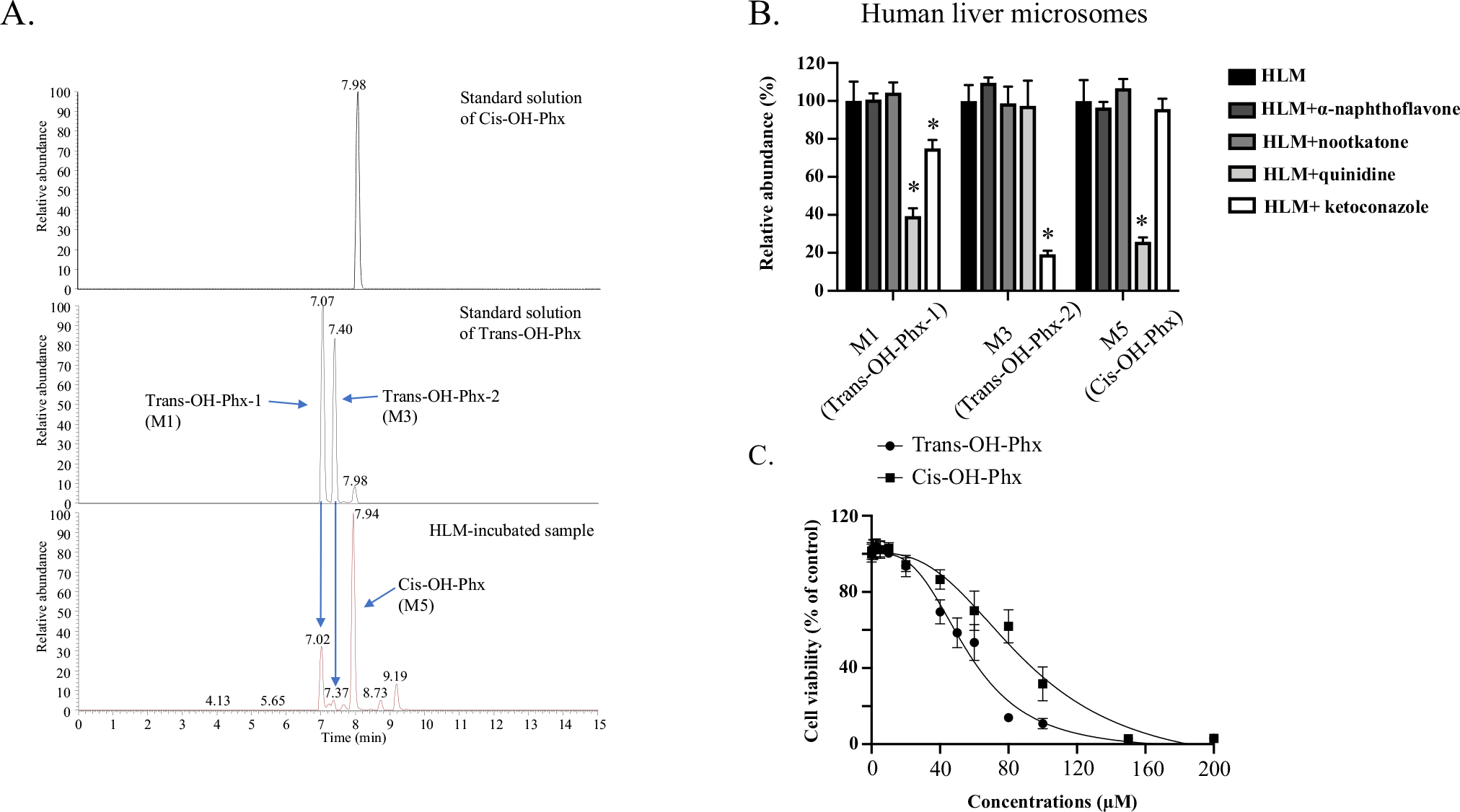
Cis-OH-Phx and trans-OH-Phx are generated by different CYPs and have different toxicity. A Representative chromatograms for perhexiline metabolites. Optimized LC–MS with a longer running time was used to better separate the multiple mono-hydroxylated metabolites, and the peaks of cis-OH-Phx (M5) and trans-OH-Phx (M1 and M3, diastereomers) were identified by the standard compound. B Perhexiline was co-incubated with the CYP1A2 inhibitor α-naphthoflavone (6 μM), the CYP2C19 inhibitor nootkatone (10 μM), the CYP2D6 inhibitor quinidine (2 μM), or the CYP3A4 inhibitor ketoconazole (2 μM) in human liver microsomes (HLM). The relative abundance of the two perhexiline metabolite M3 (trans-OH-Phx) and M5 (cis-OH-Phx) was quantified by LC–MS and normalized to the control group. *p < 0.05 compared to HLM without inhibitor. C HepG2 cells were exposed to cis-OH-Phx or trans-OH-Phx for 24 h and cell viability was measured. The results shown are mean ± SD from 3 independent experiments
