Abstract
Evaluation of tubal patency is one of the vital steps in the process of female infertility management. Hysterosalpingo-contrast Sonography (HyCoSy) has become the first-line recommendation for evaluating tubal patency. However, there remain some controversies and dilemmas relevant to the evaluation of tubal patency by HyCosy, with no effective solution has been proposed or no consensus has been reached. Herein, combined with previous research and clinical experience, we conclude and analyze these controversies and dilemmas, aiming at offering our perspective on the opportunities and challenges which are faced by HyCosy.
Keywords: Hysterosalpingo-contrast Sonography (HyCoSy), Tubal patency
International Multi-center Clinical Trial Registration Number: ChiCTR1800015519.
1. Introduction
With the development of reproductive surgery and assisted reproductive technology (ART) in recent years, it is more feasible to formulate personalized reproductive programs for infertile patients. Tubal infertility is one of the most common causes of female infertility accounting for about 30%, including different parts and different levels of tubal obstruction, peritubulitis, tubal dysfunction, and congenital tubal malformation [[1], [2], [3], [4], [5]]. Accurate evaluation of tubal status is one of the decisive reference factors for infertility management. At present, the common means to evaluate tubal patency include hysterosalpingography (HSG), hysterosalpingo-contrast sonography (HyCosy), transvaginal hydrolaparoscopy (THL), magnetic resonance-hysterosalpingography (MR-HSG), laparoscopy and dye test (LDT), and salpingoscopy [[6], [7], [8], [9], [10], [11], [12], [13]]. Since Nanani [14] et al. first applied HyCosy to assess tubal patency in 1981, HyCosy has now been widely preferred as a first-line imaging technique with the advantages of being non-radiating, non-invasive, cost-effective, and time-saving [[15], [16], [17]].
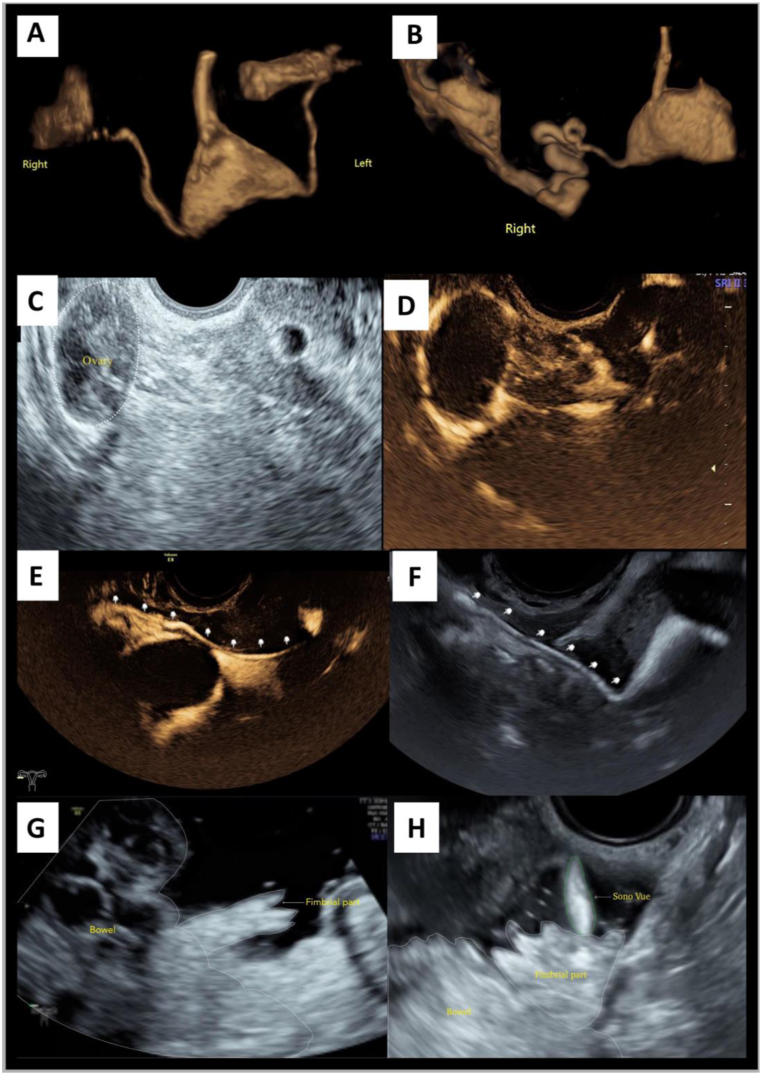
HyCosy can be divided into low mechanical index (MI) sonosalpingography and high MI sonosalpingography according to MI. The former can be further classified into dynamic three-dimensional harmonic sonosalpingography (D3D-HS), static three-dimensional harmonic sonosalpingography (S3D-HS), and two-dimensional harmonic sonosalpingography (2D-HS), while two-dimensional fundamental sonosalpingography (2D-FS) is the main mode of the latter [[18], [19], [20], [21]]. D3D-HS and S3D-HS are the two contrast modes which most cater to clinicians' reading habits (Fig. 1A and B). The female internal reproductive tracts could be sculptured by displaying positive contrast agent stereoscopically and automatically, which can significantly improve the diagnostic efficacy of HyCosy [[17], [21], [22], [23]]. Compared with S3D-HS, the advantage of D3D-HS is it has both spatial and temporal information, which let the operators observe both sides of the fallopian tubes' trajectory dynamically and simultaneously. On the other hand, S3D-HS is superior in the identification of minimal anatomical structures to D3D-HS [[23], [24], [25], [26], [27]]. Either 2D-FS or 2D-HS can track microbubble signals’ trajectory in real-time (Fig. 1C–F), the imaging would not be limited by the sampling frame, which can ensure the acquisition of distal tubal information as much as possible. Compared with 2D-HS, 2D-FS has some characteristics which harmonic sonosalpingography lacks. It is operated in a state of high mechanical index, and wave energy is normally output, so the microbubbles and surrounding anatomical structures can be displayed simultaneously.
Fig. 1.
Characteristics of different HyCosy modes (the white dotted lines represent the anatomical structure's outline, and the green dotted line represents SonoVue which overflows from the fimbrial part). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Saline infusion pelvic sonosalpingography (SIPS) is another contrast mode (Fig. 1G), by injecting negative contrast agent, i.e, normal saline into pelvic cavity via vagina-uterine-fallopian tube route (non-invasive) or colpocoeliotomia posterior route (micro-invasive), using anechoic normal saline background, the tubal surroundings and the fimbrial part's morphology and function can be observed. When combined with 2D-FS, the patency of fallopian tube may be determined intuitively by capturing the moment when the positive contrast agent overflows from the fimbrial part (Fig. 1H) [19].
A. Dynamic three-dimensional harmonic sonosalpingography (D3D-HS): Bilateral fallopian tubes are sculptured by displaying positive contrast agent dynamically, simultaneously, stereoscopically, and automatically; B. Static three-dimensional harmonic sonosalpingography (S3D-HS): Unilateral curved fallopian tubes sculptured by displaying positive contrast agent stereoscopically and automatically via a single-volume data imaging modality. The details of anatomical structures are displayed better than D3D-HS does; C-E. Two-dimensional harmonic sonosalpingography (2D-HS): Using contrast imaging simultaneous (CIS) program, two-dimensional brightness-mode ultrasonography (2D-BUS) and 2D-HS can be displayed in a dual format simultaneously. We observe the positive contrast agent encircles the ovary entirely. Using 2D-HS alone, unilateral fallopian tube can be visualized entirely in real-time (shown by the arrow); F. Two-dimensional fundamental sonosalpingography (2D-FS): The unilateral fallopian tube is visualized entirely in real-time (shown by the arrow). What's more, precise localization of the contrast agent is achieved because the microbubble signals' trajectory and its surrounding anatomical structures can be tracked simultaneously; G. Saline infusion pelvic sonosalpingography (SIPS): Taken anechoic normal saline as background, the tubal surroundings and the fimbrial part comes out more clearly; H. 2D-FS + SIPS: Combined 2D-FS with SIPS, tubal patency can be determined intuitively by capturing the moment when the positive contrast agent overflows from the fimbrial part.
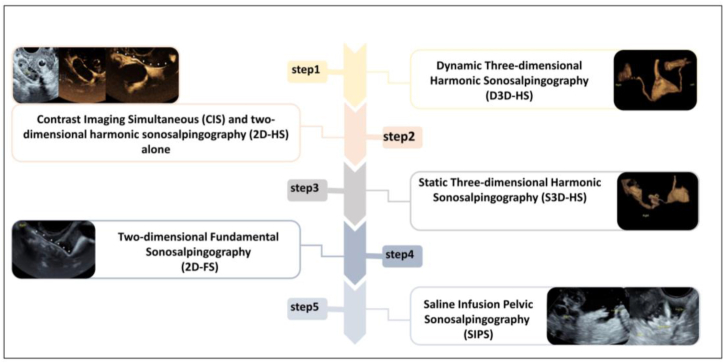
The diagnostic efficacy of HyCosy could be surely improved if all the imaging modes were combined organically [18,28]. Recommended procedures are as follows (Fig. 2): Firstly, D3D-HS should be operated to observe the whole process of contrast agent's flow from the internal reproductive tracts to the pelvic cavity; Contrast Imaging Simultaneous (CIS) program is conducted subsequently, Two-dimensional Brightness-mode Ultrasonography (2D-BUS) and 2D-HS are displayed in a dual format simultaneously to observe the ovarian encirclement and the pelvic diffusion of contrast agent; then S3D-HS is performed to collect high-resolution volume images of the fallopian tubes on both sides respectively; 2D-FS is operated after S3D-HS to explore the relationship between the contrast agent's trajectory and the surrounding anatomical structures, as an essential supplement to low mechanical index contrast modes; SIPS, or SIPS combined with 2D-FS should be performed finally, the fimbrial part's morphology and function may be observed clearly and tubal patency may be determined intuitively [19,29,30].
Fig. 2.
Recommended procedures of Hysterosalpingo-contrast Sonography (HyCoSy).
in assessment of tubal patency.
Undoubtedly, HyCosy has advantages that are difficult to be replaced with any other imaging methods. A recent systematic review analysis shows that the comprehensive sensitivity and specificity of HyCosy in assessing tubal patency are 89% and 93%, respectively [8]. However, some scholars proposed that the diagnostic efficacy of HyCosy is limited by several unresolved issues [31,32]. We used HyCoSy, hysterosalpingo-contrast sonography, and Sonosalpingography as keywords and searched out a total of 97 articles published from 2013 to 2023 in the PubMed database. The controversies and dilemmas are summarized as follows: ① The problems related to the evaluative indicators: 1) Which indicators are appropriate to be incorporated into the evaluation system have not been harmonized; 2) Some indicators have no quantitative criteria, which leads to subjective dependence inevitably; 3) The weights of various indicators lack the support in terms of evidence-based medicine. ② The definition and the grading of partial obstruction are imperfect. ③ Some situations which easily lead to false positives and false negatives have no recognized preventive methods and coping strategies. ④ The evaluation and grading of distal tubal lesions have not been fully appreciated. In this article, we'll discuss the disagreements and bottlenecks, aiming at suggesting new ideas for the development of HyCosy. To the best of our knowledge, no similar studies have been previously reported. Written informed consents are obtained from the patients for publication of the cases and accompanying images.
2. The evaluative indicators used to assess tubal patency
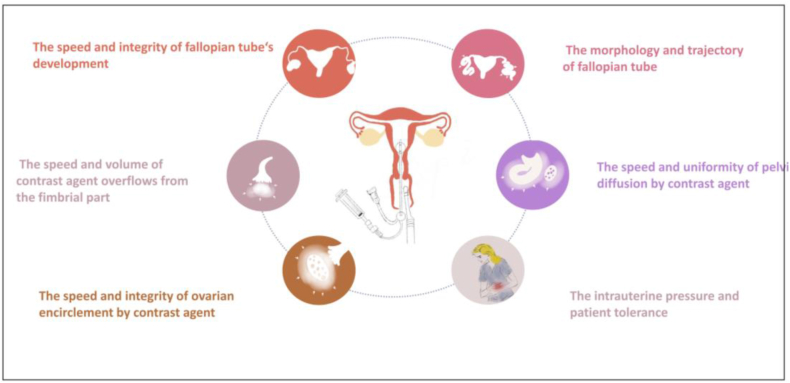
The indicators used to assess tubal patency are as follows (Fig. 3): ① The speed and integrity of fallopian tube's development; ② The speed and volume of contrast agent overflows from the fimbrial part; ③ The morphology and trajectory of fallopian tube; ④ The speed and integrity of ovarian encirclement by contrast agent; ⑤The speed and uniformity of pelvic diffusion by contrast agent; ⑥ Intrauterine pressure and patient tolerance. Diagnostic impression would be developed via the performance of all the indicators. Among these indicators, the former two are the most critical direct evidence.
Fig. 3.
The indicators of HyCosy for assessing tubal patency.
The descriptors related to tubal morphology and trajectory are as follows: naturally flexible, slender, enlarged, lengthy, twisted, coiled, angled folded, lifted, indurated, stiff, etc [33,34]. Studies have shown some of the descriptors like slender, stiff, enlarged, coiled, and indurated have good diagnostic values for different levels of obstruction [34,35]. According to our search, few studies focus on the weights of the above descriptors with large-sample and multicentric studies [34,36]. In addition, the lack of quantitative criteria for the determination of some descriptors such as slender, enlarged, lifted, lengthy, stiff, and indurated would lead to subjective judgment and result in intraobservers’ differences and different observations of the same observer [37]. It is worth mentioning that in the case of partial obstruction or complete obstruction, abnormalities of tubal morphology and trajectory can certainly play a role in corroborating speculation and improving diagnostic confidence. However, if at least one of the abnormalities coexisted in the presence of the contrast agent overflows from the fimbrial part rapidly and stably, does it mean the fallopian tube is still different from the normal and can predict an increasing risk of tubal obstruction in the physiological state? This problem is worth exploring.
In most cases, the performance of contrast agent's ovarian encirclement and pelvic diffusion contributes to judging tubal patency [17,18]. Even if no direct evidence of tubal patency on either side is obtained, the contrast agent's annular encirclement around the ovary and massive diffusion in the pelvic cavity can reliably infer good tubal patency of at least on one side [17,38]. In contrast, the absence or incomplete encirclement of the contrast agent around the ovaries, accompanied by the absence or a small amount of diffusion in the pelvic cavity can indicate that the fallopian tubes on both sides are in a state of partial obstruction, or complete obstruction [33,36]. In some special cases, the performance of ovarian encirclement needs to be analyzed prudentially. For example, a false-positive result may occur when the ovary is in the far field of the sonogram, or the fimbrial part's opening is far away and back to the ovary; a false-negative result may occur when the contrast agent remains in the enlarged ampulla and surrounds the ovary, or the contrast agent diffuses from the contralateral pelvic cavity to envelop the ovary [[38], [39], [40]]. Therefore, it is necessary to identify the relationship among the anatomical structures around the fallopian tube in the process of pre-scanning before HyCosy and to be adept at using different contrast modalities and observing from different perspectives to avoid misjudgment [34]. What should be mentioned is, from the clinical perspective, for spontaneous conception only one unobstructed tube is required. So the false-negative result goes only for the tube (left or right), not for the examination. Detecting the contrast agent encircles the ovary or diffuses in the pelvic shows the examination's result is negative, namely, at least one of the tubes is unobstructed.
The value of intrauterine pressure and patient tolerance in predicting tubal patency has not yet been determined. Intrauterine pressure mainly depends on tubal patency, the expansion degree and the fluid capacity of tubal distal part, the injection bolus volume and speed of contrast agent, the volume and speed of cervico-vaginal reflux, the volume and speed of venous reflux, the ratio of catheter balloon's size to uterine capacity, etc [37]. Patients' tolerance is closely associated with intrauterine pressure, their individualized mental status and threshold of pain, etc [[41], [42], [43]]. Partial obstruction or complete obstruction is only one of the factors which lead to increased intrauterine pressure or intolerance in patients [13]. Therefore, whether these two indicators are suitable to be routine diagnostic basis or not for speculating tubal patency is open to question.
3. Definition and grading of partial obstruction
Some scholars divide tubal patency into 3 types: patency, partial obstruction, and complete obstruction [18,33]. Patency and complete obstruction have clear definitions respectively. Partial obstruction refers to the state between patency and complete obstruction [38]. There have ambiguous views on how to define partial obstruction from the perspective of HyCosy [33]. Given this, other scholars cancel the diagnosis of partial obstruction and retain the other two: patency and complete obstruction [31,44]. We believe partial obstruction exists objectively, which may affect every conceptive step that need to be completed in the fallopian tube such as egg pick-up, sperm capacitation, sperm-egg combination and fertilized egg delivery, resulting in infertility or tubal pregnancy. Different levels of partial obstruction are proven to be relevant to different rates of natural intrauterine pregnancy and different occurrence risks of tubal pregnancy [35]. Hence, the definition and the grading of partial obstruction can provide information for clinical strategies. For instance, expectant treatment should be given to infertile women who have the possibility of natural conception; ART should be recommended for those who have completely lost the function of fallopian tubes and have no significance for salpingoplasty; for patients with indications for salpingoplasty, HyCosy's evaluative result is beneficial for the choice of timing and modalities of reproductive surgeries [45].
Some sonographic findings are used to define partial obstruction, which can be divided into direct signs and indirect signs [[17], [18], [19],30,34]. The former shows the full development of fallopian tube but a slow development rate; a small amount of contrast agent overflows from the fimbrial part slowly or sprays from the fimbrial part like a spike under pressurized injection. The latter is characterized by the abnormal morphology and trajectory of fallopian tube (including the fimbrial part); a small amount of contrast agent surrounds the unilateral ovary incompletely and diffuses in the unilateral pelvic cavity; the signs suggestive of increased intrauterine pressure such as increased injection resistance, repeated rotation of contrast agent in the uterine cavity, cervico-vaginal reflux, and patient's intolerance. According to literature review and clinical experience, we consider the direct signs can be used as independent factors for diagnosing partial obstruction, while the value of indirect signs are uncertain.
Employing Hysterosalpingography (HSG), some scholars have established a grading system for partial obstruction into 3 levels: mild, moderate, and severe, according to the degree of tubal patency, the trajectory of fallopian, the morphology of tubal mucosal folds, the performance of contrast agent overflows from the fimbrial part and diffuses into the pelvic cavity [45]. However, there has few study focus on the partial obstruction's grading or scoring system by using HyCosy according to our search. In order to evaluate the exact degree of tubal lesions and predict the prognosis of reproductive surgery more accurately, we believe it is required to draw on the experience of HSG's grading system, using LDT as the golden standard to establish a HyCosy grading or scoring system for partial obstruction.
4. False positives and false negatives of HyCosy
Evaluation of tubal patency by HyCosy has a certain percentage of false positives and false negatives. Before discussing this issue, it should be recognized that since HyCosy is performed in a perfused state, the diagnostic impression obtained cannot represent tubal patency in a physiological state. Moreover, tubal patency alone cannot completely assess whether the fallopian tube affects pregnancy or not because good tubal patency can also affect conception if the function of egg pick-up or fertilized egg delivery were impaired [18].
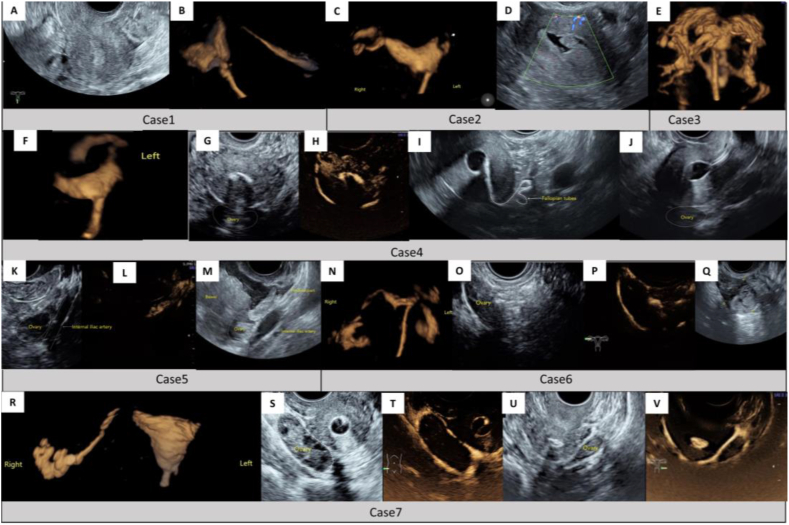
The causes of misjudgment can be summarized as follows (Fig. 4): ① The loss of the anatomical information of the fallopian tube, especially the information of the contrast agent's overflow from the fimbrial part, due to the trajectory of the fallopian tube, inexperienced operator, the limit of equipment's performance, or inappropriate presetting, etc. ② Pseudo obstruction of the proximal part of fallopian tube, due to various factors such as tubal spasm, intrauterine lesions, and the improper indwelling of catheter, etc [31,[46], [47], [48]]. According to statistics, the false positive rate of HSG in the diagnosis of proximal obstruction is about 16%–40% [49]. In another study, HSG showed that 60% of the patients with proximal obstruction have good tubal patency unexpectedly after one month, without any treatment performed [50]. ③ Massive para-uterine venous reflux (PUVR): PUVR is closely related to adenomyosis, history of uterine cavity surgery, thin endometrium, poor endometrial repair, endometrial injury during the catheterization, and so on [31,32,[51], [52], [53]]. On the one hand, if a massive contrast agent is shunted into a lower-pressure venous system rapidly in advance, it may cause delayed visualization or no visualization of the fallopian tubes, resulting in false positives; On the other hand, if PUVR and the fallopian tubes were overlapped by each other on the images, it might be difficult to identify the real fallopian tube, resulting in false negatives or false positives [31,51]. ④ The artifact of ovarian encasement, as mentioned earlier [40].
Fig. 4.
Cases of false positives and false negatives in the assessment of tubal patency by HyCosy.
Case 1 (A, B): When the uterus is at the mean position (A), the proximal part of the left fallopian tube is prone to be lost during the process of D3D-HS or S3D-HS (B). Case 2 (C-D): The whole left fallopian tube cannot be displayed by D3D-HS (C). Thus we speculate that the proximal part of the left fallopian tube may be obstructed completely. At the same time, we also note the ipsilateral cornu uteri has not been filled with contrast agent (shown by the arrow). When SIPS is performed right after (D), several endometrial polypus in the left cornu uteri are displayed on the transverse section of the uterine fundus, which leads to the conclusion of pseudo-obstruction. Case 3 (E): Massive and quick para-uterine venous reflux (PUVR) has occurred during the process of D3D-HS. Both sides of the fallopian tubes are submerged in a mess. Case 4 (F-J): The distal part of the left fallopian tube has not been captured by D3D-HS (F), and the left ovary (Shown by the white dotted line) is displayed without the encirclement of contrast agent by CIS (G, H). Based on the imaging information above, we speculate that the distal part may be completely obstructed. However, when 2D-FS is performed (I), the whole course of the left fallopian tube and the overflow of contrast agent from the fimbrial part is displayed in real-time. To explain the inconsistency, we review the 2D-BUS images and find the left ovary is located in the sonogram's far field and adjacent to the uterus (on the longitudinal section of the uterus, shown by the white dotted line) (J), which leads to a false-positive result. Case 5 (K-M) (the white dotted lines represent the anatomical structure's outline): This case failed to capture D3D-HS video the first time, we turn to conduct CIS and find the left ovary is displayed without the contrast agent's envelop (K, L), which drops a hint that the ipsilateral fallopian tube may be obstructed. However, the left fallopian tube is proven to be patency by 2D-FS. To explain the inconsistency, we perform SIPS subsequently and find the left fimbrial part's opening is far away and back to the left ovary, dynamic scanning shows that the positive contrast agent overflows from the fimbrial part, and the overflow direction is away from the ovary (M). Case 6 (N-Q): The whole process of the right fallopian tube is displayed by D3D-HS. It seems as if the contrast agent overflowed from the fimbrial part and encircled the ipsilateral ovary (N). CIS then also produces an artifact of ovarian encasement, which leads to a diagnostic trap (shown by the white dotted line) (O, P). Meanwhile, we find the right pelvic cavity has no contrast agent diffusion. By using 2D-FS, we find the distal part of the right fallopian tube is atretic and adheres to the ovary, the contrast agent is remained in the enlarged ampulla and surrounds the ovary (Q). Case 7 (R–V): During the process of D3D-HS, the right fallopian tube is diagnosed as patency while the left one is not displayed (R); CIS is conducted subsequently which shows both sides of the ovaries are wrapped by the contrast agent (shown by the white dotted line) (S–V). Accordingly, we speculate the left fallopian tube may have good patency, but its imaging information is lost by D3D-HS. Laparoscopic dye test (LDT) is performed later and finds the right fallopian tube has good patency, while the left one is completely obstructed. Thus, we conclude the contrast agent overflows from the right fimbrial part, diffuses to the whole pelvic cavity and envelopes the left ovary, which leads to a pitfall.
Most of the misjudgments can be easily identified and corrected by optimized processes and experienced operators, while some of the others can not. Some scholars have recommended several preventing methods or coping strategies as follows: ① When the uterus is at the mean position, or the ovary is located in the sonogram's far field and adjacent to the uterus, or the fallopian tube is raised vertically, the trajectory of the fallopian tube is not conducive to be observed via transvaginal route [30,31,39]. Some scholars have suggested a solution for transabdominal approach assessment [39]. ② Fallopian tube spasm is one of the main causes leading to tubal pseudo obstruction. Methods to prevent tubal spasms include psychological concerns, application of anesthetics, gentle operation, reduction of the duration of intubation, and proximity of the injected fluid temperature to body temperature [41,54,55].③ PUVR's incidence rate is 27.9%–41.3%. By optimizing the operating procedures in advance, such as improving the skills of catheterization, and injecting the contrast agent into the fallopian tube directly by selective tubal catheterization, PUVR's incidence rate can be reduced to a certain extent. Some scholars have also presented some coping strategies once PUVR occurs. For instance, tracking down the whole fallopian tube from the Cornus uteri; looking for the fimbrial part around the ipsilateral ovary; observing the moment of contrast agent overflowing from the fimbrial part directly by SIPS, etc [18,19]. However, these measures can only deal with part of the cases and their actual efficiency still need to be verified by large-sample and multicentric studies.
5. Evaluation and grading of distal tubal lesions
Fimbrial part is closely related to the key step of egg picking. Tubal distal part's partial obstruction and peri-fimbria adhesion are the main risk factors leading to tubal infertility [56,57]. The evaluation and grading of distal tubal lesions are vital for selecting clinical strategies and predicting postoperative prognosis, with an evidence level of 2C [[58], [59], [60]]. Therefore, evaluation of the distal tubal lesions, such as the constrictive fimbrial part, accessory fimbrial part, fimbrial part's accessory openings, fimbrial part's mucosal bridge, etc., has become an important procedure in the management of female infertility.
The American Society for Reproductive Medicine (ASRM) has established a scoring system for distal tubal lesions based on the degree of tubal dilatation, tubal wall thickness, the proportion of the fimbrial part's mucosal folds, and the extent and density of surrounding adhesions seen during the laparoscopy [60]. In the field of imaging examination, HSG has many contributions to the preoperative evaluation of distal tubal lesions [61,62], while the ultrasonic value has not been fully appreciated. Some scholars proposed the application of SIPS combined with 2D-FS or three-Dimensional HyCoSy (i.e., S3D-HS) or four-dimensional hysterosalpingo-contrast sonography (i.e., D3D-HS) to observe the fimbrial part's morphology and function, including the brightness-mode characteristics of the fimbrial part and the overflow characteristics of contrast agent from the fimbrial part [19,29,30]. However, the actual value of HyCosy for evaluating and grading distal tubal lesions still needs further exploration. We expect the advantages of SIPS should be fully utilized in the future, and the weights of various ultrasonic indicators related to distal tubal lesions should be determined, using LDT as the gold standard. In this way, a comprehensive, objective, and operable grading system for distal tubal lesions by HyCosy can be established, matching the corresponding scoring system recommended by ASRM.
6. Conclusions
The incidence of tubal infertility is increasing in recent years. Clinicians in the reproductive field are increasingly looking for more individualized reproductive programs for tubal infertile patients. Higher requirements are put forward for the evaluative efficiency of tubal patency. Although HyCosy has been confirmed by a large number of clinical practices as a valuable imaging technique with good prospects for assessing tubal patency it is still confronted with a number of controversies and dilemmas which limit its diagnostic value. How to maximize the strengths of HyCosy and treat the limitations of HyCosy rationally? The proposition need further exploration and discussion.
What should be emphasized finally is that the clinicians must combine the result of HyCosy with the information of patient's age, ovarian reservation, history of pregnancy and delivery, other infertility factors, history of gynecological surgery, history of infectious diseases, and the site of fallopian tube injury, etc [63,64]. Thereby, a reliable reference can be acquired for the determination of reproductive programs.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Medical and Health Technology Project in Zhejiang Province of China (No.2020KY430), the Excellent Scientific Research Foundation of Zhejiang Provincial People's Hospital (No. ZRY2021B012).
References
- 1.Farquhar C.M., Bhattacharya S., Repping S., Mastenbroek S., Kamath M.S., Marjoribanks J., Boivin J. Female subfertility. Nat. Rev. Dis. Prim. 2019;5(1):7. doi: 10.1038/s41572-018-0058-8. [DOI] [PubMed] [Google Scholar]
- 2.Carson S.A., Kallen A.N. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76. doi: 10.1001/jama.2021.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briceag I., Costache A., Purcarea V.L., Cergan R., Dumitru M., Briceag I., Sajin M., Ispas A.T. Fallopian tubes--literature review of anatomy and etiology in female infertility. J Med Life. 2015;8(2):129–131. [PMC free article] [PubMed] [Google Scholar]
- 4.Thurston L., Abbara A., Dhillo W.S. Investigation and management of subfertility. J. Clin. Pathol. 2019;72(9):579–587. doi: 10.1136/jclinpath-2018-205579. [DOI] [PubMed] [Google Scholar]
- 5.Khalaf Y. ABC of subfertility. Tubal subfertility,BMJ. 2003;327(7415):610–613. doi: 10.1136/bmj.327.7415.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dishuck C.F., Perchik J.D., Porter K.K., Gunn D.D. Advanced imaging in female infertility. Curr. Urol. Rep. 2019;20(11):77. doi: 10.1007/s11934-019-0942-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X.Y., Cai-Hong M.A. The Application of Transvaginal Hydrolaparoscopy in Reproductive Medicine. Journal of International Reproductive Health/Family Planning. 2017;36(3):185–188, 225. doi: 10.3969/j.issn.1674-1889.2017.03.002. [DOI] [Google Scholar]
- 8.Chen L.S., Zhu Z.Q., Li J., Wang Z.T., Qiang Y., Hu X.Y., Zhang M.M., Wang Z.Q. Hysterosalpingo-contrast-sonography vs. magnetic resonance-hysterosalpingography for diagnosing fallopian tubal patency: a systematic review and meta-analysis. Eur. J. Radiol. 2020;125 doi: 10.1016/j.ejrad.2020.108891. [DOI] [PubMed] [Google Scholar]
- 9.Vitale S.G., Carugno J., Riemma G., Torok P., Cianci S., De Franciscis P., Parry J.P. Hysteroscopy for assessing fallopian tubal obstruction: a systematic review and diagnostic test accuracy meta-analysis. J. Minim. Invasive Gynecol. 2021;28(4):769–778. doi: 10.1016/j.jmig.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Bohiltea R.E., Mihai B.M., Stanica C.D., Gheorghe C.M., Berceanu C., Dima V., Bohiltea A.T., Neagu S., Vladareanu R. Technical tips and tricks after 10 Years of HyFoSy for tubal patency testing. J. Clin. Med. 2022;11(19) doi: 10.3390/jcm11195946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos J., Pellicer N., Fernandez-Sanchez M. Hysterosalpingography is obsolete: hysterosalpingo-contrast foam sonography should be the alternative. Reprod. Biomed. Online. 2022;45(5):839–842. doi: 10.1016/j.rbmo.2022.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Lorincz J., Vitale S.G., Barna S.K., Dinko F., Riemma G., Tunde H., Nagyhazi O., Lampe R., De Franciscis P., Torok P. Hystero-salpingo scintigraphy for fallopian tubal patency assessment: results from a prospective study. Minim Invasive Ther. Allied Technol. 2022;31(5):797–802. doi: 10.1080/13645706.2021.1986845. [DOI] [PubMed] [Google Scholar]
- 13.Torok P., Molnar S., Herman T., Jashanjeet S., Lampe R., Riemma G., Vitale S.G. Fallopian tubal obstruction is associated with increased pain experienced during office hysteroscopy: a retrospective study. Updates Surg. 2020;72(1):213–218. doi: 10.1007/s13304-020-00712-x. [DOI] [PubMed] [Google Scholar]
- 14.Nannini R., Chelo E., Branconi F., Tantini C., Scarselli G.F. Dynamic echohysteroscopy: a new diagnostic technique in the study of female infertility. Acta Eur. Fertil. 1981;12(2):165–171. [PubMed] [Google Scholar]
- 15.Expert I., Panel on Women's. Wall D.J., Reinhold C., Akin E.A., Ascher S.M., Brook O.R., Dassel M., Henrichsen T.L., Learman L.A., Maturen K.E., Patlas M.N., Robbins J.B., Sadowski E.A., Saphier C., Uyeda J.W. P. Glanc,ACR appropriateness criteria(R) female infertility. J. Am. Coll. Radiol. 2020;17(5S):S113–S124. doi: 10.1016/j.jacr.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Lin X.N., Huang G.N., Sun H.X., Fan L.Q., Feng Y., Shen H., Liu P., Hong L.W. Zhang Y.S.,Wang X.X,Chinese expert consensus on diagnosis and treatment of tubal infertility. Journal of Reproductive Medicine. 2018;27(11):1048–1056. doi: 10.3969/j.issn.1004-3845.2018.11.002. [DOI] [Google Scholar]
- 17.Grigovich M., Kacharia V.S., Bharwani N., Hemingway A., Mijatovic V., Rodgers S.K. Evaluating fallopian tube patency: what the radiologist needs to know. Radiographics. 2021;41(6):1876–18961. doi: 10.1148/rg.2021210033. [DOI] [PubMed] [Google Scholar]
- 18.Chen S., Du X., Chen Q., Chen S. Combined real-time three-dimensional hysterosalpingo-contrast sonography with B mode hysterosalpingo-contrast sonography in the evaluation of fallopian tube patency in patients undergoing infertility investigations. Biomed Res Int.2019. 2019 doi: 10.1155/2019/9408141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z., Peng C., Lv Y., Sun J., Chen S., Cui A., Jin B. The performance of transvaginal two-dimensional fundamental sonosalpingography combined with saline infusion pelvic sonosalpingography for assessing fimbrial part's morphology and function of the fallopian tubes. J. Ultrasound Med. 2022;41(1):41–50. doi: 10.1002/jum.15677. [DOI] [PubMed] [Google Scholar]
- 20.Yu J., Cai M., Liang W., Deng Z., Xie Y. Diagnostic efficacy of 3-D hysterosalpingo-contrast sonography in the detection of tubal occlusion: systematic meta-analysis. J. Obstet. Gynaecol. Res. 2015;41(9):1418–1425. doi: 10.1111/jog.12728. [DOI] [PubMed] [Google Scholar]
- 21.Pei R. Comparison of effectiveness as well as advantages and disadvantages of different dimensions of hysterosalpingo-contrast sonography for diagnosis of lesions associated with female infertility. Comput Math Methods Med.2022. 2022 doi: 10.1155/2022/7508880. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Alcazar J.L., Martinez A., Duarte M., Welly A., Marin A., Calle A., Garrido R., Pascual M.A., Guerriero S. Two-dimensional hysterosalpingo-contrast-sonography compared to three/four-dimensional hysterosalpingo-contrast-sonography for the assessment of tubal occlusion in women with infertility/subfertility: a systematic review with meta-analysis. Hum. Fertil. 2022;25(1):43–55. doi: 10.1080/14647273.2020.1769204. [DOI] [PubMed] [Google Scholar]
- 23.Gu P., Yang X., Zhao X., Xu D. The value of transvaginal 4-dimensional hysterosalpingo-contrast sonography in predicting the necessity of assisted reproductive technology for women with tubal factor infertility. Quant Imaging Med Surg. 2021;11(8):3698–3714. doi: 10.21037/qims-20-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen F., Quan J., Huang P., You X. Hysterosalpingo-contrast sonography with four-dimensional technique for screening fallopian tubal patency: let's make an exploration. J. Minim. Invasive Gynecol. 2017;24(3):407–414. doi: 10.1016/j.jmig.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Kong D., Dong X., Wang Z., Zhang L., Shao X., Qi Y. Four-dimensional hysterosalpingo-contrast sonography with auxiliary hydrogen peroxide examination for the diagnosis of fallopian tube patency following interventional treatment of ovarian ectopic cysts. Arch. Gynecol. Obstet. 2017;295(2):519–526. doi: 10.1007/s00404-016-4259-7. [DOI] [PubMed] [Google Scholar]
- 26.Alcazar J.L., Martinez-Astorquiza Corral T., Orozco R., Dominguez-Piriz J., Juez L., Errasti T. Three-dimensional hysterosalpingo-contrast-sonography for the assessment of tubal patency in women with infertility: a systematic review with meta-analysis. Gynecol. Obstet. Invest. 2016;81(4):289–295. doi: 10.1159/000443955. [DOI] [PubMed] [Google Scholar]
- 27.He Y., Geng Q., Liu H., Han X. First experience using 4-dimensional hysterosalpingo-contrast sonography with SonoVue for assessing fallopian tube patency. J. Ultrasound Med. 2013;32(7):1233–1243. doi: 10.7863/ultra.32.7.1233. [DOI] [PubMed] [Google Scholar]
- 28.Exacoustos C., Di Giovanni A., Szabolcs B., Binder-Reisinger H., Gabardi C., Arduini D. Automated sonographic tubal patency evaluation with three-dimensional coded contrast imaging (CCI) during hysterosalpingo-contrast sonography (HyCoSy) Ultrasound Obstet. Gynecol. 2009;34(5):609–612. doi: 10.1002/uog.7442. [DOI] [PubMed] [Google Scholar]
- 29.Wang W., Zhou Q., Gong Y., Li Y., Huang Y., Chen Z. Assessment of fallopian tube fimbria patency with 4-dimensional hysterosalpingo-contrast sonography in infertile women. J. Ultrasound Med. 2017;36(10):2061–2069. doi: 10.1002/jum.14244. [DOI] [PubMed] [Google Scholar]
- 30.Hong Q., Cai R., Chen Q., Zhang S., Ai A., Fu Y., Kuang Y. Three-dimensional HyCoSy with perfluoropropane-albumin microspheres as contrast agents and normal saline injections into the pelvic cavity for morphological assessment of the fallopian tube in infertile women. J. Ultrasound Med. 2017;36(4):741–748. doi: 10.7863/ultra.16.03041. [DOI] [PubMed] [Google Scholar]
- 31.Liang N., Wu Q.Q., Li J.H., Gao F.Y., Sun F.L., Guo C.X. Causes of misdiagnosis in assessing tubal patency by transvaginal real-time three-dimensional hysterosalpingo-contrast sonography. Rev. Assoc. Med. Bras. 2019;65(8):1055–1060. doi: 10.1590/1806-9282.65.8.1055. [DOI] [PubMed] [Google Scholar]
- 32.Shi J., Li S., Wu H., He Y., Yi W., Xu J., Liu H., Guan Y. The influencing factors of venous intravasation during transvaginal four-dimensional hysterosalpingo-contrast sonography with SonoVue. Ultrasound Med. Biol. 2019;45(9):2273–2280. doi: 10.1016/j.ultrasmedbio.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Li J., Yu L., Han S., Shen X., Jia X. Application of 3D-HyCoSy in the diagnosis of oviduct obstruction. Exp. Ther. Med. 2017;13(3):966–970. doi: 10.3892/etm.2017.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S., Li Y., Qi C. Evaluation of Tubal Patency by Transvaginal Reconstructive Three-dimensional Hysterosalpingo-contrast Sonography. Chinese Journalof Ultrasound in Medicing. 2010;26(10):932–934. doi: 10.3969/j.issn.1002-0101.2010.10.026. [DOI] [Google Scholar]
- 35.Liu Y., Zhang N., He Y., Shi J., Zhou M., Xu J., Liu H. Spontaneous conception outcome in infertile women after four-dimensional hysterosalpingo-contrast-sonography. BMC Pregnancy Childbirth. 2020;20(1):638. doi: 10.1186/s12884-020-03315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Q., Wang S.S., Zhu X.S., Li F. Evaluation of tubal patency with transvaginal three-dimensional hysterosalpingo-contrast sonography. Chin. Med. Sci. J. 2015;30(2):70–75. doi: 10.1016/s1001-9294(15)30014-6. [DOI] [PubMed] [Google Scholar]
- 37.Qiang Y., Wu Y., Cai T. Clinical significance of increasing pressure curve's slope when injecting ultrasound contrast agent during evaluation of fallopian tubal patency. J. Ultrasound Med. 2021;40(11):2329–2338. doi: 10.1002/jum.15616. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L., Zhang X., Chen X., Liao L., Pan R., Zhou N., Di N. Value of three-dimensional hysterosalpingo-contrast sonography with SonoVue in the assessment of tubal patency. Ultrasound Obstet. Gynecol. 2012;40(1):93–98. doi: 10.1002/uog.11085. [DOI] [PubMed] [Google Scholar]
- 39.Zheng W.W., Chen L., Chen S., Zhu J., Lin F., Xu S. Value of ovarian positional assessment on 4D hysterosalpingo-contrast sonography. Med Ultrason. 2022;24(2):167–173. doi: 10.11152/mu-3209. [DOI] [PubMed] [Google Scholar]
- 40.He Y., Ma X., Xu J., Li S., Wu H., Liu Q., Kong L., Luo J., Liu H. Comparison of assessment methods for fallopian tubal patency and peritubal adhesion between transvaginal 4-dimensional hysterosalpingo-contrast sonography and laparoscopic chromopertubation. J. Ultrasound Med. 2017;36(3):547–556. doi: 10.7863/ultra.15.11056. [DOI] [PubMed] [Google Scholar]
- 41.Zhang N., Liu Y., He Y., Shi J., Zhou M., Liu H. Transvaginal four-dimensional hysterosalpingo-contrast sonography: pain perception and factors influencing pain severity. J. Obstet. Gynaecol. Res. 2021;47(1):302–310. doi: 10.1111/jog.14538. [DOI] [PubMed] [Google Scholar]
- 42.Boned-Lopez J., Alcazar J.L., Errasti T., Ruiz-Zambrana A., Rodriguez I., Pascual M.A., Guerriero S. Severe pain during hysterosalpingo-contrast sonography (HyCoSy): a systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021;304(6):1389–1398. doi: 10.1007/s00404-021-06188-3. [DOI] [PubMed] [Google Scholar]
- 43.Maxim A.R., Gligor O.H., Badea R.I. Comparison of Hystero-salpingography and Hysterosalpingo-Contrast Sonography for tubal patency testing: technical success, pain perception, side effects and complications. Med Ultrason. 2021;23(3):283–288. doi: 10.11152/mu-2692. [DOI] [PubMed] [Google Scholar]
- 44.Lanzani C., Savasi V., Leone F.P.G., Ratti M., Ferrazzi E. Two-dimensional HyCoSy with contrast tuned imaging technology and a second-generation contrast media for the assessment of tubal patency in an infertility program. Fertil. Steril. 2009;92(3):1158–1161. doi: 10.1016/j.fertnstert.2008.07.1746. [DOI] [PubMed] [Google Scholar]
- 45.Xu H., Tang J. Advances in clinical and prognostic research of Hysterosalpingo-contrast Sonography. Chin. J. Clin. 2015;43(12):21–24. [Google Scholar]
- 46.Sun Y., Zhang J., Bai W. Higher prevalence of endometrial polyps in patients with fallopian tube obstruction: a case-control study. J. Minim. Invasive Gynecol. 2019;26(5):935–940. doi: 10.1016/j.jmig.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Hajishafiha M., Zobairi T., Zanjani V.R., Ghasemi-Rad M., Yekta Z., Mladkova N. Diagnostic value of sonohysterography in the determination of fallopian tube patency as an initial step of routine infertility assessment. J. Ultrasound Med. 2009;28(12):1671–1677. doi: 10.7863/jum.2009.28.12.1671. [DOI] [PubMed] [Google Scholar]
- 48.Abrao M.S., Muzii L., Marana R. Anatomical causes of female infertility and their management. Int. J. Gynaecol. Obstet. 2013;123(Suppl 2):S18–S24. doi: 10.1016/j.ijgo.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Saunders R.D., Shwayder J.M., Nakajima S.T. Current methods of tubal patency assessment. Fertil. Steril. 2011;95(7):2171–2179. doi: 10.1016/j.fertnstert.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 50.Dessole S., Meloni G.B., Capobianco G., Manzoni M.A., Ambrosini G., Canalis G.C. A second hysterosalpingography reduces the use of selective technique for treatment of a proximal tubal obstruction. Fertil. Steril. 2000;73(5):1037–1039. doi: 10.1016/s0015-0282(00)00415-5. [DOI] [PubMed] [Google Scholar]
- 51.He Y., Wu H., Xiong R., Liu H., Shi J., Xu J., Zhang N., Liu Y. Intravasation affects the diagnostic image quality of transvaginal 4-dimensional hysterosalpingo-contrast sonography with SonoVue. J. Ultrasound Med. 2019;38(8):2169–2180. doi: 10.1002/jum.14914. [DOI] [PubMed] [Google Scholar]
- 52.Wang W., Zhou Q., Zhou X., Chen Z., Zhang H. Influence factors on contrast agent venous intravasation during transvaginal 4-dimensional hysterosalpingo-contrast sonography. J. Ultrasound Med. 2018;37(10):2379–2385. doi: 10.1002/jum.14594. [DOI] [PubMed] [Google Scholar]
- 53.Ludwin A., Ludwin I., Martins W.P. Venous intravasation during evaluation of tubal patency by ultrasound contrast imaging. Ultrasound Obstet. Gynecol. 2018;51(1):143–145. doi: 10.1002/uog.17405. [DOI] [PubMed] [Google Scholar]
- 54.Fenzl V. Effect of different ultrasound contrast materials and temperatures on patient comfort during intrauterine and tubal assessment for infertility. Eur. J. Radiol. 2012;81(12):4143–4145. doi: 10.1016/j.ejrad.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Nirmal D., Griffiths A.N., Jose G., Evans J. Warming Echovist contrast medium for hysterocontrastsonography and the effect on the incidence of pelvic pain. A randomized controlled study. Hum. Reprod. 2006;21(4):1052–1054. doi: 10.1093/humrep/dei440. [DOI] [PubMed] [Google Scholar]
- 56.Famurewa O., Adeyemi A., Ibitoye O., Ogunsemoyin O. Association between history of abdominopelvic surgery and tubal pathology. Afr. Health Sci. 2013;13(2):441–446. doi: 10.4314/ahs.v13i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyons R.A., Saridogan E., Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update. 2006;12(4):363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- 58.Suresh Y.N., Narvekar N.N. The role of tubal patency tests and tubal surgery in the era of assisted reproductive techniques. Obstet. Gynaecol. 2014;16(1):37–45. [Google Scholar]
- 59.Daniilidis A., Balaouras D., Chitzios D., Theodoridis T., Assimakopoulos E. Hydrosalpinx: tubal surgery or in vitro fertilisation? An everlasting dilemma nowadays; a narrative review. J Obstet Gynaecol. 2017;37(5):550–556. doi: 10.1080/01443615.2017.1287685. [DOI] [PubMed] [Google Scholar]
- 60.Practice A.a. o. Committee of the American Society for Reproductive Medicine. Electronic address,Role of tubal surgery in the era of assisted reproductive technology: a committee opinion. Fertil. Steril. 2021;115(5):1143–1150. doi: 10.1016/j.fertnstert.2021.01.051. [DOI] [PubMed] [Google Scholar]
- 61.Kahyaoglu S., Yumusak O.H., Kahyaoglu I., Kucukbas G.N., Esercan A., Tasci Y. Evaluation of time lapse for establishing distal tubal occlusion diagnosis during hysterosalpingography procedure performed by using water soluble contrast media. J. Chin. Med. Assoc. 2017;80(5):313–318. doi: 10.1016/j.jcma.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Guan J., Watrelot A. Fallopian tube subtle pathology. Best Pract. Res. Clin. Obstet. Gynaecol. 2019;59:25–40. doi: 10.1016/j.bpobgyn.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Guan J. Reproductive surgery and microsurgery of the fallopian tubes in assisted reproductive technology. Chin. J. Clin. 2015;9(1):1–7. [Google Scholar]
- 64.Practice A.a. o. Committee of the American society for reproductive medicine. Electronic address and M. Practice committee of the American society for Reproductive,Fertility evaluation of infertile women: a committee opinion. Fertil. Steril. 2021;116(5):1255–1265. doi: 10.1016/j.fertnstert.2021.08.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.