Abstract
Leucine-rich glioma-inactivated 1 (LGI1) was identified as a causative gene of autosomal dominant lateral temporal lobe epilepsy. We previously reported that Lgi1-mutant rats carrying a missense mutation (L385R) showed audiogenic seizure-susceptibility. To explore the pathophysiological mechanisms underlying Lgi1-related epilepsy, we evaluated changes in glutamate and GABA release in Lgi1-mutant rats. Acoustic priming (AP) for audiogenic seizure-susceptibility was performed by applying intense sound stimulation (130 dB, 10 kHz, 5 min) on postnatal day 16. Extracellular glutamate and GABA levels in the hippocampus CA1 region were evaluated at 8 weeks of age, using in vivo microdialysis techniques. Under naïve conditions without AP, glutamate and GABA release evoked by high-K+ depolarization was more prominent in Lgi1-mutant than in wild-type (WT) rats. The AP treatment on day 16 significantly increased basal glutamate levels and depolarization-induced glutamate release both in Lgi1-mutant and WT rats, yielding greater depolarization-induced glutamate release in Lgi1-mutant rats. On the other hand, the AP treatment enhanced depolarization-induced GABA release only in WT rats, and not in Lgi1-mutant rats, illustrating reduced GABAergic neurotransmission in primed Lgi1-mutant rats. The present results suggest that enhanced glutamatergic and reduced GABAergic neurotransmission are involved in the audiogenic seizure-susceptibility associated with Lgi1-mutation.
Keywords: Epilepsy, Lgi1, ADLTE, Audiogenic seizure, Glutamate, GABA
1. Introduction
Epilepsy is a chronic disease characterized by recurrent unprovoked seizures associated with neural hyperexcitation, affecting about 1% of the population worldwide [1]. Although epilepsy-related mutations are often located in genes of ion channels and ligand-gated channels, diverse pathogenic mutations in genes encoding non-ion channel proteins have also been identified [2]. Among them, heterozygous mutations of Leucine-rich glioma-inactivated 1 (LGI1) were first reported to cause autosomal dominant lateral temporal lobe epilepsy (ADLTE, OMIM 600512), an inherited epileptic syndrome characterized by partial seizures with predominant auditory symptoms (e.g., auditory auras and seizure-susceptibility to specific sounds) [[3], [4], [5]].
Lgi1 is a neural secreted protein highly expressed in the central nervous system, especially in the hippocampus and neocortex [6]. Lgi1 interacts with a disintegrin and metalloproteinase (ADAM) family (e.g., ADAM22 and ADAM23) and post synaptic density protein 95 (PSD-95), and this interaction regulates the function of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors and voltage-gated K+ (Kv) 1.1 channels in synapses [[7], [8], [9], [10], [11]]. Moreover, auto-antibodies against LGI1 have been detected in patients with autoimmune limbic encephalitis presenting with frequent seizures and memory deficits [[12], [13], [14]]. However, the mechanisms underlying Lgi1-related epilepsy remain elusive.
We previously developed a rat model that carries a missense mutation (L385R) of the Lgi1 gene with ENU (N-ethyl-N-nitrosourea) mutagenesis [15]. This mutation impaired Lgi1 secretion, which was similar to the most of missense mutations responsible for ADLTE [15]. Furthermore, the Lgi1-mutant rats showed seizure-susceptibility to sound stimuli, sharing similarity with the clinical features of ADLTE [[15], [16], [17]]. Namely, Lgi1-mutant rats, which received acoustic priming (AP) treatments (130 dB, 10 kHz, 5 min) on postnatal day (P) 16, showed generalized tonic-clonic seizures (GTCSs) following wild running behaviors (sensitized startle responses) by acoustic test stimulation (130 dB, 10 kHz, 1 min) at 8 weeks of age. However, primed wild-type (WT) rats only exhibited wild running behaviors with the test stimulation, but no GTCSs [[15], [16], [17]]. In addition, we showed that astrocytic inwardly rectifying K+ (Kir) 4.1 channels, which regulate the clearance mechanism of excessive extracellular K+ (spatial K+ buffering) and glutamate transport into astrocytes [[18], [19], [20]], were down-regulated in the Lgi1-mutant rats, suggesting that alterations in extracellular K+ and/or glutamate levels are involved in audiogenic epileptogenesis [17,21].
In this study, we evaluated synaptic glutamate and GABA release in the hippocampus, which expresses a high density of Lgi1 [6,22], using audiogenic seizure-susceptible Lgi1-mutant rats to explore the mechanisms underlying the pathogenesis of Lgi1-related epilepsy.
2. Materials and methods
2.1. Animals and ethical approval
F344-Lgi1m1Kyo rats (NBRP Rat No. 0656) with a heterozygous missense mutation (L385R/+) by N-ethyl-N-nitrosourea (ENU) mutagenesis techniques were provided by the National BioResource Project-Rat (NBRP-Rat, http://www.anim.med.kyoto-u.ac.jp) [15]. The animals were bred and maintained according to the animal care methods complying with the Guide for the Care and Use of Laboratory Animals of the Ministry of Education, Science, Sports and Culture of Japan. All experimental procedures in this study were approved by the Animal Research Committee of Kyoto University and Osaka Medical and Pharmaceutical University (formerly Osaka University of Pharmaceutical Sciences). Only male animals were used for neurotransmission analyses.
2.2. Genotyping
F344-Lgi1m1Kyo rats were mated with their WT littermates. DNA extracted from blood samples of rats on P15 was used for genotyping using a PCR-restriction fragment length polymorphism technique, as described previously [17]. Briefly, exon 8 including the mutation site was amplified by PCR using the Ampdirect Plus PCR buffer (Shimadzu, Japan). PCR products were digested with Xsp I (Takara Bio, Japan) and discriminated between mutant allele and wild-type allele using conventional agarose gel electrophoresis.
2.3. Acoustic priming (AP) treatments for audiogenic seizure generation
AP stimulation at P16 confers audiogenic seizure susceptibility on Lgi1-mutant rats, which evokes GTCSs with test stimulation at 8 weeks of age [17]. AP treatments for audiogenic seizure generation were performed using previously reported methods [[15], [16], [17]]. Briefly, animals were individually placed in a plastic cage (17 × 25 × 13 cm) within a larger sound-proof box and, after 1-min habituation, an intense AP stimulation (130 dB, 10 kHz, 5 min) was given by a loudspeaker (JBL Professional) placed centrally on the cover of the cage. Tone bursts were generated by a sound stimulator (DPS-725, Dia Medical System Co.) and amplified using a power amplifier (D75-A, Amcron). Non-primed animals were handled in the same manner described above, but no AP stimulation was delivered. All animals were then subjected to in vivo microdialysis studies at 8 weeks of age without receiving acoustic test stimulation.
2.4. In vivo microdialysis
Lgi1-mutant or WT rats at 7 weeks were anesthetized with pentobarbital (40 mg/kg, i. p.) and fixed in a stereotaxic instrument (Narishige, SR-6, Japan). A guide cannula was inserted into a position 1 mm above the hippocampus CA1 region (2.0 mm lateral and 3.8 mm caudal to the bregma, and 2.0 mm deep from the cortical surface) [23] and fixed to the skull with dental cement. After a recovery period of about one week, animals were subjected to in vivo microdialysis study, as previously reported [24]. A dialysis probe (Eicom, A-I-6-01, Japan) was inserted into the hippocampus CA1 region through a guide cannula and artificial cerebrospinal fluid (aCSF), containing NaCl 140 mM, KCl 2.4 mM, MgCl2 1.0 mM, CaCl2 1.2 mM, and NaHCO3 5.0 mM, was perfused at a flow rate of 1.5 μL/min using a microperfusion pump (Fig. 1A). High concentration K+ (50 mM)-containing aCSF was perfused for 60 min to evaluate the depolarization-induced synaptic release. The dialysate samples were collected every 10 min. After experiments, animals were deeply anesthetized with an intraperitoneal injection of pentobarbital (80 mg/kg) and the brain was removed from the skull. Then, 100-μm-thick coronal sections were prepared from each brain using a microslicer and the position of each injection site was checked.
Fig. 1.
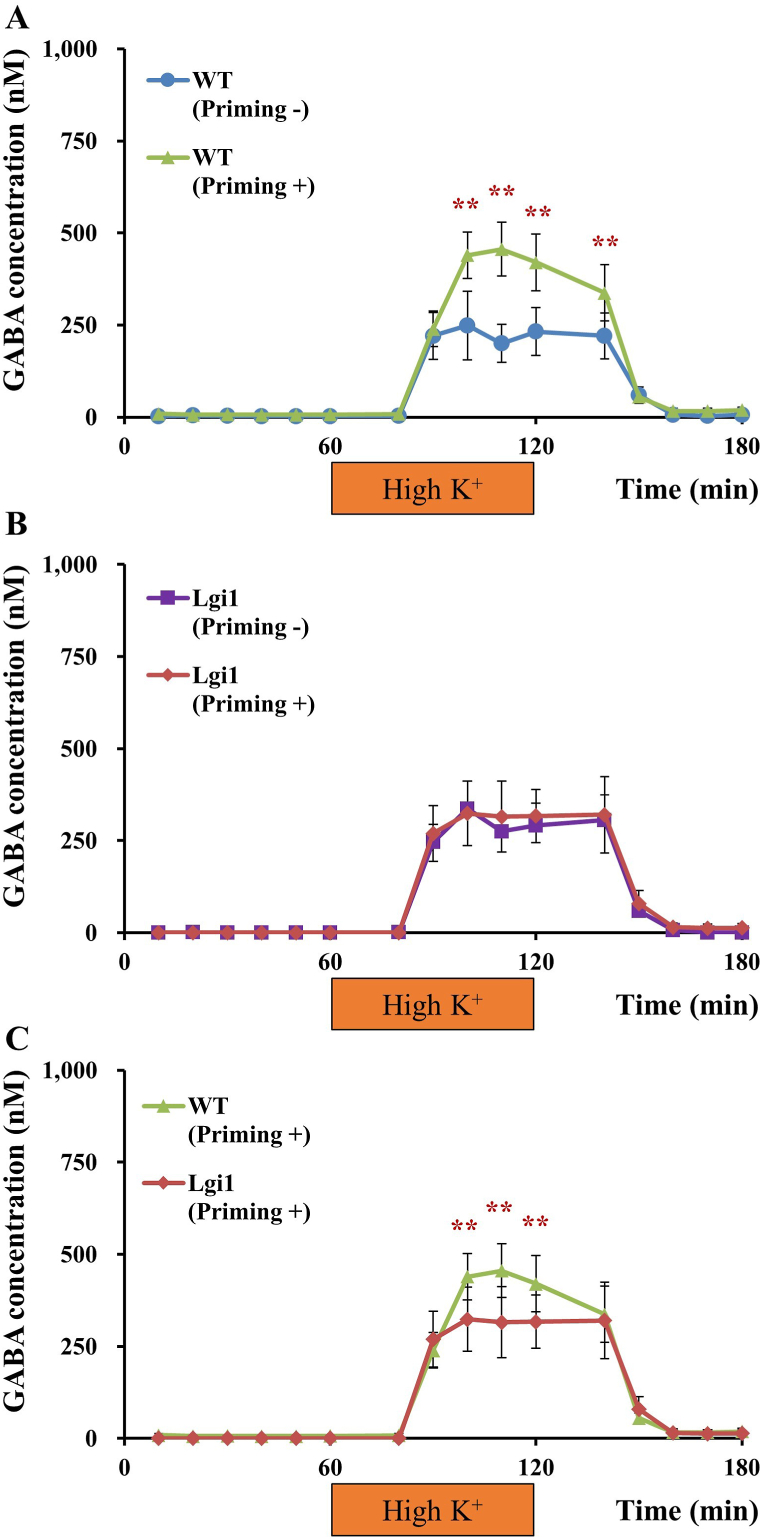
Schematic overview and experimental flow chart of in vivo microdialysis techniques (A). Glutamate and GABA release in the hippocampus. Extracellular levels of glutamate (B) and GABA (C) were compared between wild-type (WT) rats and Lgi1-mutant rats. Depolarization stimulation involved applying high-concentration K+ (50 mM)-containing artificial cerebrospinal fluid (aCSF) for 60 min through the dialysis probe. Each point represents the mean ± S.E.M. of 5 or 6 animals. *P < 0.05, **P < 0.01, significantly different from WT group.
2.5. Quantification of glutamate and GABA with HPLC
The dialysate samples were analyzed for glutamate and GABA levels using a HPLC-ECD system. Briefly, glutamate and GABA were derivatized with o-phthalaldehyde and separated on a cation exchange column (Eicom, 3.0 mm φ × 150 mm Eicompak SC-5ODS, Japan). The mobile phase consisted of 0.1 M phosphate buffer, 5 mg/L EDTA 2Na, pH 6.0, with 27% methanol pumped at a flow rate of 500 μL/min. The area under the curve for glutamate and GABA peaks were measured using eDAQ Power Chrom (eDAQ Pty Ltd., Australia). Extracellular glutamate and GABA values were quantified with external standard curves generated by four standard concentrations (10 nM, 100 nM, 1 μM, and 10 μM).
2.6. Statistical analysis
All data are expressed as the mean ± S.E.M. The significance of differences among multiple groups was determined by two-way ANOVA followed by Tukey's post-hoc test. A P-value of less than 0.05 was considered significant.
3. Results
In this study, Lgi1-mutant and WT rats were divided into two groups, respectively, according to whether they received AP stimulation (130 dB, 10 kHz, 5 min) on P16 as follows, non-primed Lgi1-mutant rats (n = 5), primed Lgi1-mutant rats (n = 5), non-primed WT rats (n = 6), and primed WT rats (n = 7). We then conducted in vivo microdialysis experiments at 8 weeks of age to evaluate extracellular glutamate and GABA levels in the hippocampal CA1 region (Fig. 1A). No animals were exposed to acoustic test stimulation to avoid behavioral influences (e.g., wild running or seizures) on glutamate and GABA levels.
Under naïve conditions without AP treatments, there was no difference in basal extracellular levels of glutamate or GABA between Lgi1-mutant and WT rats (Fig. 1B and C). However, high K+ (50 mM)-induced release of both glutamate and GABA was significantly enhanced in Lgi1-mutant rats.
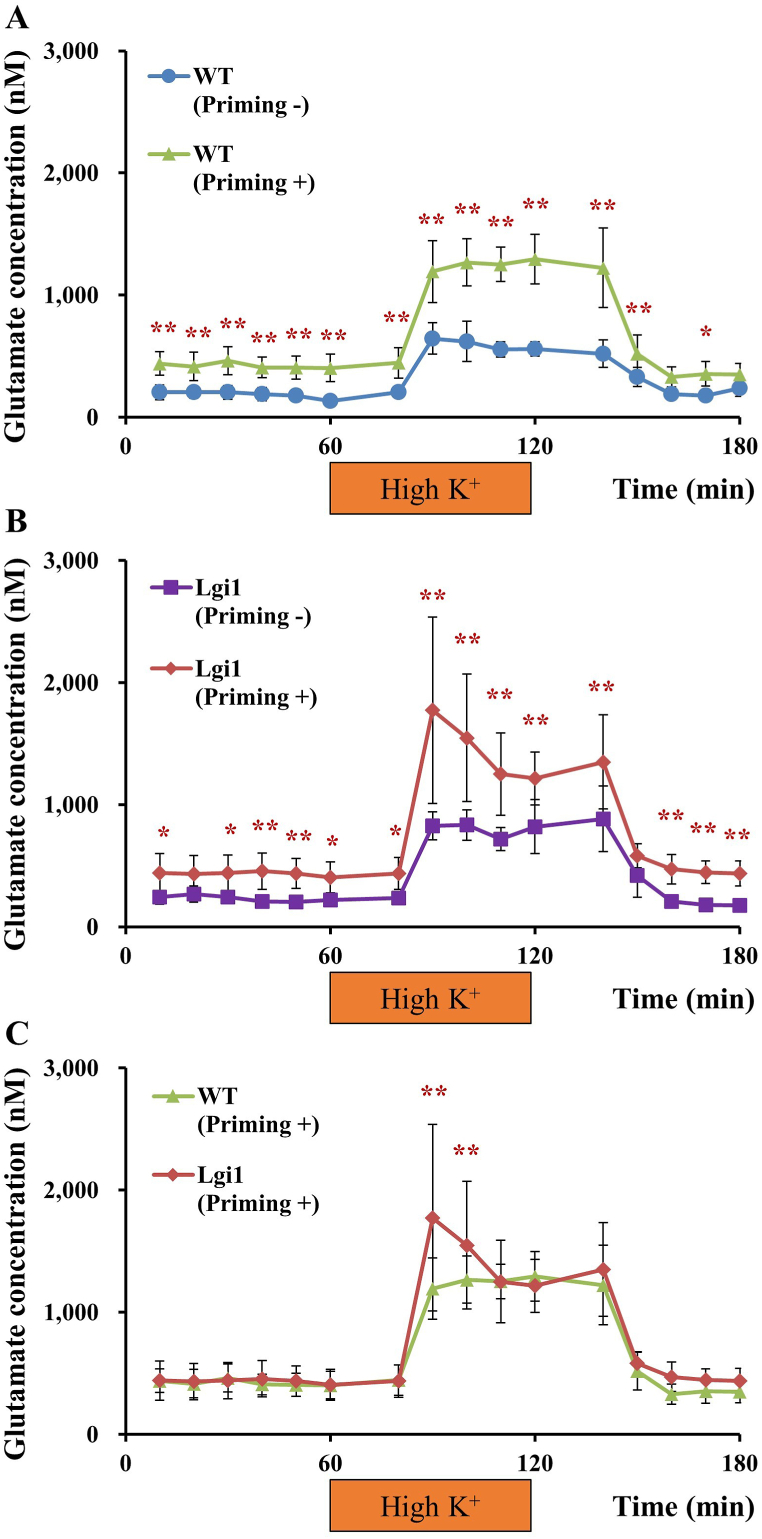
Treatments of animals with AP stimulation on P16 significantly elevated basal levels of glutamate both in Lgi1-mutant and WT rats (Fig. 2A and B). High K+-induced glutamate release was also enhanced by AP treatments in both groups (Fig. 2A and B), yielding greater depolarization-induced glutamate release in Lgi1-mutant rats (Fig. 2C). In contrast, AP treatments did not affect basal GABA levels in Lgi1-mutant or WT rats (Fig. 3A and B). In addition, AP treatments significantly enhanced high K+-induced GABA release only in WT rats, and not in Lgi1-mutant rats (Fig. 3A and B), illustrating that depolarization-induced GABA release was more prominent in WT than in Lgi1-mutant rats (Fig. 3C).
Fig. 2.
Glutamate release in the hippocampus. Extracellular levels of glutamate were compared between non-primed wild-type (WT) and primed WT rats (A), non-primed Lgi1-mutant and primed Lgi1-mutant rats (B), and primed WT and primed Lgi1-mutant rats (C) (data from Fig. 1B redisplayed for comparisons between non-primed rats and primed rats). Depolarization stimulation involved applying high-concentration K+ (50 mM)-containing artificial cerebrospinal fluid (High K+) for 60 min through the dialysis probe. Each point represents the mean ± S.E.M. of 5–7 animals. *P < 0.05, **P < 0.01, significantly different from each other group.
Fig. 3.
GABA release in the hippocampus. Extracellular levels of GABA were compared between non-primed wild-type (WT) and primed WT rats (A), non-primed Lgi1-mutant and primed Lgi1-mutant rats (B), and primed WT and primed Lgi1-mutant rats (C) (data from Fig. 1C redisplayed for comparisons between non-primed rats and primed rats). Depolarization stimulation involved applying high-concentration K+ (50 mM)-containing artificial cerebrospinal fluid (High K+) for 60 min. Each point represents the mean ± S.E.M. of 5–7 animals. **P < 0.01, significantly different from each other group.
4. Discussion
Emerging evidence shows that the impairment of Lgi1, a secreted protein that regulates glutamatergic synaptic transmission, is associated with genetic and autoimmune epilepsy [5,10,11,14]. Here, we demonstrated for the first time that depolarization-induced synaptic release of glutamate and GABA markedly changed in Lgi1-mutant rats, a rat model of human ADLTE (summarized in Fig. 4A).
Fig. 4.
Summarized results on hippocampal glutamate and GABA release in Lgi1-mutant rats (A) and schematic drawing on the imbalance of glutamatergic and GABAergic neurotransmission potentially linked to audiogenic seizure-susceptibility in Lgi1-mutant rats (B). Under naïve (non-primed) conditions, the Lgi1-mutation potentiated high K+ depolarization-evoked glutamate and GABA release. On the other hand, acoustic priming (AP) stimulation (at P16) increased the basal glutamate level both in Lgi1-mutant and WT rats. It should be noted that, under primed condition, the Lgi1-mutation caused enhanced glutamate release and diminished GABA release during high K+ depolarization (shown in yellow). This imbalance of glutamatergic and GABAergic neurotransmission in primed Lgi1-mutant rats may be involved in generation of audiogenic seizures associated with Lgi1-mutation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
AP treatments given in the juvenile stage (P16) cause audiogenic excitatory behaviors (e.g., wild running and jumping) at a mature age (8 weeks of age) in WT rats. However, these animals rarely exhibit any convulsive seizures. In contrast, most animals carrying the loss-of-function mutation (L385R) develop GTCSs following wild running/jumping behaviors with acoustic test stimulation [[15], [16], [17]], suggesting that the dysfunction of Lgi1 facilitates audiogenic epileptogenesis. The present study demonstrated that AP stimulation applied on P16 significantly elevated basal levels of extracellular glutamate and enhanced depolarization-induced synaptic release of glutamate not only in Lgi1-mutant rats, but also in WT rats. Thus, developmental changes leading to hyper-excitation of glutamatergic neurotransmission may be involved in generation of audiogenic excitatory behaviors (e.g., wild running and jumping) after juvenile AP stimulation. On the other hand, depolarization-induced glutamate release in primed Lgi1-mutant rats was more prominent than in primed WT rats. In addition, although AP treatments also increased depolarization-induced GABA release in WT rats, this response to AP was abolished in Lgi1-mutant rats. Thus, the imbalance of excitatory/inhibitory neurotransmission, enhanced glutamate and reduced GABA synaptic release, in the hippocampus is supposed to cause more severe seizures such as GTCSs in Lgi1-mutant rats (Fig. 4B). Since LGI1-related glutamatergic transmission is reportedly involved in regulation of postsynaptic GABAergic interneurons in the hippocampus [10,11,25], dysfunction of Lgi1 might fail to activate GABAergic neurons.
Even under naïve conditions without AP treatments, depolarization-induced glutamate and GABA release was more prominent in Lgi1-mutant rats than in WT rats. Our results on the enhancement of depolarization-induced glutamate release by the Lgi1 mutation are consistent with the previous finding that truncated mutant Lgi1 increased the spine density and enhanced excitatory transmission [26]. Although the mechanism for the increase of evoked GABA release in Lgi1-mutant rats remains uncertain, this might be a compensatory response to the enhanced glutamate release.
While audiogenic seizures are reportedly associated with increased glutamate in the inferior colliculus and midbrain nucleus of the auditory pathway in other rodent models [27,28], we previously demonstrated that the hippocampus was one of the audiogenic seizure foci in Lgi1-mutant rats, where astrocytic Kir4.1 channels were significantly down-regulated during the development of epileptogenesis [16,17]. Astrocytic Kir4.1 channels mediate spatial K+ buffering and glutamate uptake into astrocytes via EAAT1 and EAAT2 [[18], [19], [20], [21],29,30], playing a crucial role in the development of epilepsy [21]. Thus, down-regulation of Kir4.1 channels might at least partly be involved in the elevation of hippocampal glutamate level in Lgi1-mutant rats. In addition, we showed that the extracellular signal-regulated kinase (ERK) signaling pathway was activated by the inhibition of astrocytic Kir4.1 channels, which facilitated the secretion of brain-derived neurotrophic factor (BDNF) from astrocytes [31]. Since the development of audiogenic seizures and other epileptic seizures is known to be associated with increased BDNF-tropomyosin receptor kinase B (TrkB) signaling and ERK signaling [[32], [33], [34], [35], [36], [37], [38], [39]], down-regulated Kir4.1 channels might also facilitate audiogenic epileptogenesis in Lgi1-mutation via the ERK/BDNF pathway. However, further studies are necessary to delineate the mechanisms underlying the interaction of Lgi1 with Kir4.1 channels and BDNF.
In conclusion, we analyzed synaptic release of glutamate and GABA in the hippocampus of Lgi1-mutant rats, where Lgi1 secretion was impaired [15]. The present study suggests that depolarization-induced synaptic release of glutamate was potentiated, but that of GABA release was diminished by the Lgi1-mutation. This imbalance of glutamatergic and reduced GABAergic neurotransmission may be involved in the audiogenic seizure-susceptibility associated with Lgi1-mutation. However, since the present results were obtained solely by in vivo microdialysis study, further analyses of functional changes by the Lgi1-mutation are necessary, especially the Lgi1 interaction with glutamate and GABA neurons using electrophysiological as well as immunohistochemical techniques, to delineate the mechanisms underlying Lgi1-related epilepsies.
Author contribution statement
Masato Kinboshi, Yukihiro Ohno: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Saki Shimizu, Kentaro Tokudome: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tomoji Mashimo, Tadao Serikawa, Hidefumi Ito, Ryosuke Takahashi, Akio Ikeda: Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Akio Ikeda is a professor and head of the Department of Epilepsy, Movement Disorders and Physiology (Industry-Academia Collaboration Courses) supported by Eisai Co., Ltd., Nihon Kohden Corporation, Otsuka Pharmaceutical Co., and UCB Japan Co., Ltd. All other authors declare no conflicts of interest.
Acknowledgments
We would like to thank the National BioResource Project - Rat (http://www.anim.med.kyoto-u.ac.jp/NBR/) for providing rat strains (F344-Lgi1m1Kyo, No 0656).
References
- 1.Ngugi A.K., Bottomley C., Kleinschmidt I., et al. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers C.T., Mefford H.C. Advancing epilepsy genetics in the genomic era. Genome Med. 2015;7:91. doi: 10.1186/s13073-015-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalachikov S., Evgrafov O., Ross B., et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat. Genet. 2002;30:335–341. doi: 10.1038/ng832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morante-Redolat J.M., Gorostidi-Pagola A., Piquer-Sirerol S., et al. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum. Mol. Genet. 2002;11:1119–1128. doi: 10.1093/hmg/11.9.1119. [DOI] [PubMed] [Google Scholar]
- 5.Nobile C., Michelucci R., Andreazza S., et al. LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum. Mutat. 2009;30:530–536. doi: 10.1002/humu.20925. [DOI] [PubMed] [Google Scholar]
- 6.Senechal K.R., Thaller C., Noebels J.L. ADPEAF mutations reduce levels of secreted LGI1, a putative tumor suppressor protein linked to epilepsy. Hum. Mol. Genet. 2005;14:1613–1620. doi: 10.1093/hmg/ddi169. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y., Oses-Prieto J., Kim M.Y., et al. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J. Neurosci. 2010;30:1038–1048. doi: 10.1523/JNEUROSCI.4661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovero K.L., Fukata Y., Granger A.J., et al. The LGI1-ADAM22 protein complex directs synapse maturation through regulation of PSD-95 function. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E4129–E4137. doi: 10.1073/pnas.1511910112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petit-Pedrol M., Sell J., Planagumà J., et al. LGI1 antibodies alter Kv1.1 and AMPA receptors changing synaptic excitability, plasticity and memory. Brain. 2018;141:3144–3159. doi: 10.1093/brain/awy253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fels E., Muñiz-Castrillo S., Vogrig A., et al. Role of LGI1 protein in synaptic transmission: from physiology to pathology. Neurobiol. Dis. 2021;160 doi: 10.1016/j.nbd.2021.105537. [DOI] [PubMed] [Google Scholar]
- 11.Fukata Y., Hirano Y., Miyazaki Y., et al. Trans-synaptic LGI1-ADAM22-MAGUK in AMPA and NMDA receptor regulation. Neuropharmacology. 2021;194 doi: 10.1016/j.neuropharm.2021.108628. [DOI] [PubMed] [Google Scholar]
- 12.Lai M., Huijbers M.G., Lancaster E., et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani S.R., Alexander S., Waters P., et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramanathan S., Al-Diwani A., Waters P., Irani S.R. The autoantibody-mediated encephalitides: from clinical observations to molecular pathogenesis. J. Neurol. 2021;268:1689–1707. doi: 10.1007/s00415-019-09590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baulac S., Ishida S., Mashimo T., et al. A rat model for LGI1-related epilepsies. Hum. Mol. Genet. 2012;21:3546–3557. doi: 10.1093/hmg/dds184. [DOI] [PubMed] [Google Scholar]
- 16.Fumoto N., Mashimo T., Masui A., et al. Evaluation of seizure foci and genes in the Lgi1(L385R/+) mutant rat. Neurosci. Res. 2014;80:69–75. doi: 10.1016/j.neures.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Kinboshi M., Shimizu S., Mashimo T., et al. Down-regulation of astrocytic Kir4.1 channels during the audiogenic epileptogenesis in Leucine-rich glioma-Inactivated 1 (Lgi1) mutant rats. Int. J. Mol. Sci. 2019;20:1013. doi: 10.3390/ijms20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucheryavykh Y.V., Kucheryavykh L.Y., Nichols C.G., et al. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia. 2007;55:274–281. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- 19.Djukic B., Casper K.B., Philpot B.D., et al. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen M.L., Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J. Neurochem. 2008;107:589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinboshi M., Ikeda A., Ohno Y. Role of astrocytic inwardly rectifying potassium (Kir) 4.1 channels in epileptogenesis. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.626658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herranz-Pérez V., Olucha-Bordonau F.E., Morante-Redolat J.M., et al. Regional distribution of the leucine-rich glioma inactivated (LGI) gene family transcripts in the adult mouse brain. Brain Res. 2010;1307:177–194. doi: 10.1016/j.brainres.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G., Watson C. sixth ed. Elsevier; New York: 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 24.Tokudome K., Okumura T., Terada R., et al. A missense mutation of the gene encoding synaptic vesicle glycoprotein 2A (SV2A) confers seizure susceptibility by disrupting amygdalar synaptic GABA release. Front. Pharmacol. 2016;7:210. doi: 10.3389/fphar.2016.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukata Y., Yokoi N., Miyazaki Y., Fukata M. The LGI1-ADAM22 protein complex in synaptic transmission and synaptic disorders. Neurosci. Res. 2017;116:39–45. doi: 10.1016/j.neures.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y.D., Lee S., Jin Z., et al. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat. Med. 2009;15:1208–1214. doi: 10.1038/nm.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribak C.E., Byun M.Y., Ruiz G.T., Reiffenstein R.J. Increased levels of amino acid neurotransmitters in the inferior colliculus of the genetically epilepsy-prone rat. Epilepsy Res. 1988;2:9–13. doi: 10.1016/0920-1211(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 28.Ross K.C., Coleman J.R. Developmental and genetic audiogenic seizure models: behavior and biological substrates. Neurosci. Biobehav. Rev. 2000;24:639–653. doi: 10.1016/s0149-7634(00)00029-4. [DOI] [PubMed] [Google Scholar]
- 29.Kofuji P., Newman E.A. Potassium buffering in the central nervous system. Neuroscience. 2004;129:1045–1056. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hibino H., Inanobe A., Furutani K., et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 31.Kinboshi M., Mukai T., Nagao Y., et al. Inhibition of inwardly rectifying potassium (Kir) 4.1 channels facilitates brain-derived neurotrophic factor (BDNF) expression in astrocytes. Front. Mol. Neurosci. 2017;10:408. doi: 10.3389/fnmol.2017.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binder D.K., Routbort M.J., Ryan T.E., et al. Selective inhibition of kindling development by intraventricular administration of TrkB receptor body. J. Neurosci. 1999;19:1424–1436. doi: 10.1523/JNEUROSCI.19-04-01424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottschalk W.A., Jiang H., Tartaglia N., et al. Signaling mechanisms mediating BDNF modulation of synaptic plasticity in the hippocampus, Learn. Memoir. 1999;6:243–256. [PMC free article] [PubMed] [Google Scholar]
- 34.Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barton M.E., Shannon H.E. The seizure-related phenotype of brain-derived neurotrophic factor knockdown mice. Neuroscience. 2005;136:563–569. doi: 10.1016/j.neuroscience.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich C., Lähteinen S., Suzuki F., et al. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol. Dis. 2011;42:35–47. doi: 10.1016/j.nbd.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Liu G., Gu B., He X.P., et al. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron. 2013;79:31–38. doi: 10.1016/j.neuron.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glazova M.V., Nikitina L.S., Hudik K.A., et al. Inhibition of ERK1/2 signaling prevents epileptiform behavior in rats prone to audiogenic seizures. J. Neurochem. 2015;132:218–229. doi: 10.1111/jnc.12982. [DOI] [PubMed] [Google Scholar]
- 39.Chernigovskaya E.V., Korotkov A.A., Dorofeeva N.A., et al. Delayed audiogenic seizure development in a genetic rat model is associated with overactivation of ERK1/2 and disturbances in glutamatergic signaling. Epilepsy Behav. 2019;99 doi: 10.1016/j.yebeh.2019.106494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.