Figure 1.
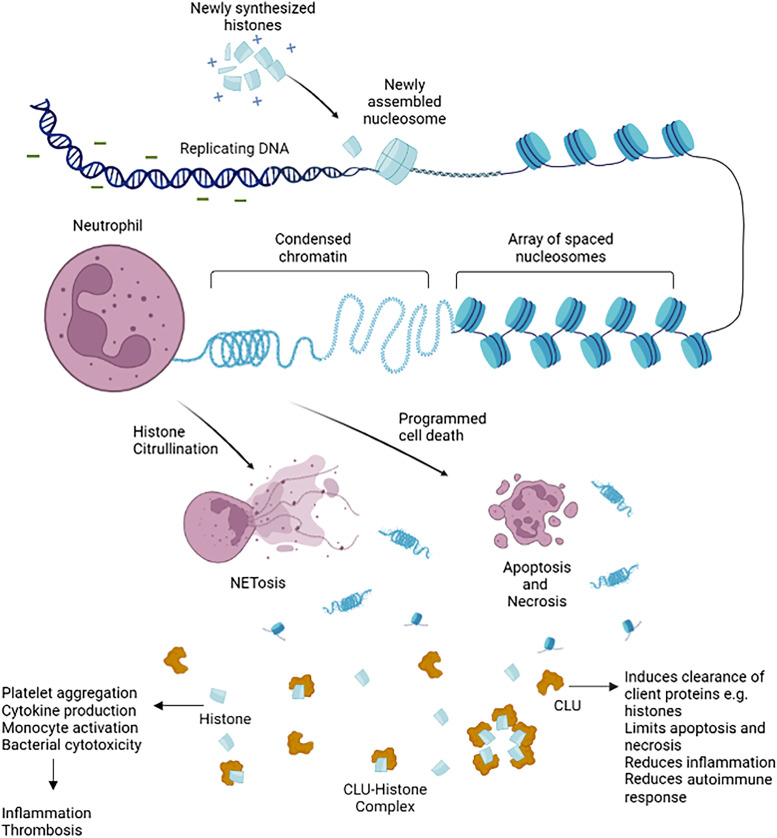
Histones are basic proteins whose positive charges associate with DNA, enabling the negatively charged DNA to spool around them. They form memberships of chromatin beads called nucleosomes, with each nucleosome bead consisting of a histone octamer composed of two copies of each histone protein: H2A, H2B, H3, and H4. They are coiled within the cell, forming condensed chromatin material. Extracellular histones are released by cell death in response to infection and inflammation as chromatin material, nucleosome, or individual histones. CLU (Clusterin), an extracellular chaperone protein, forms bonds to misfolded or excess proteins, including histones, forming complexes. CLU–histone complexes neutralize histone activity. In addition, these complexes aid the clearance of extracellular histones by binding to cell surface receptor(s); they are internalized by receptor-mediated endocytosis and trafficked to autophagosomes for degradation. NET = neutrophil extracellular trap.