Figure 3.
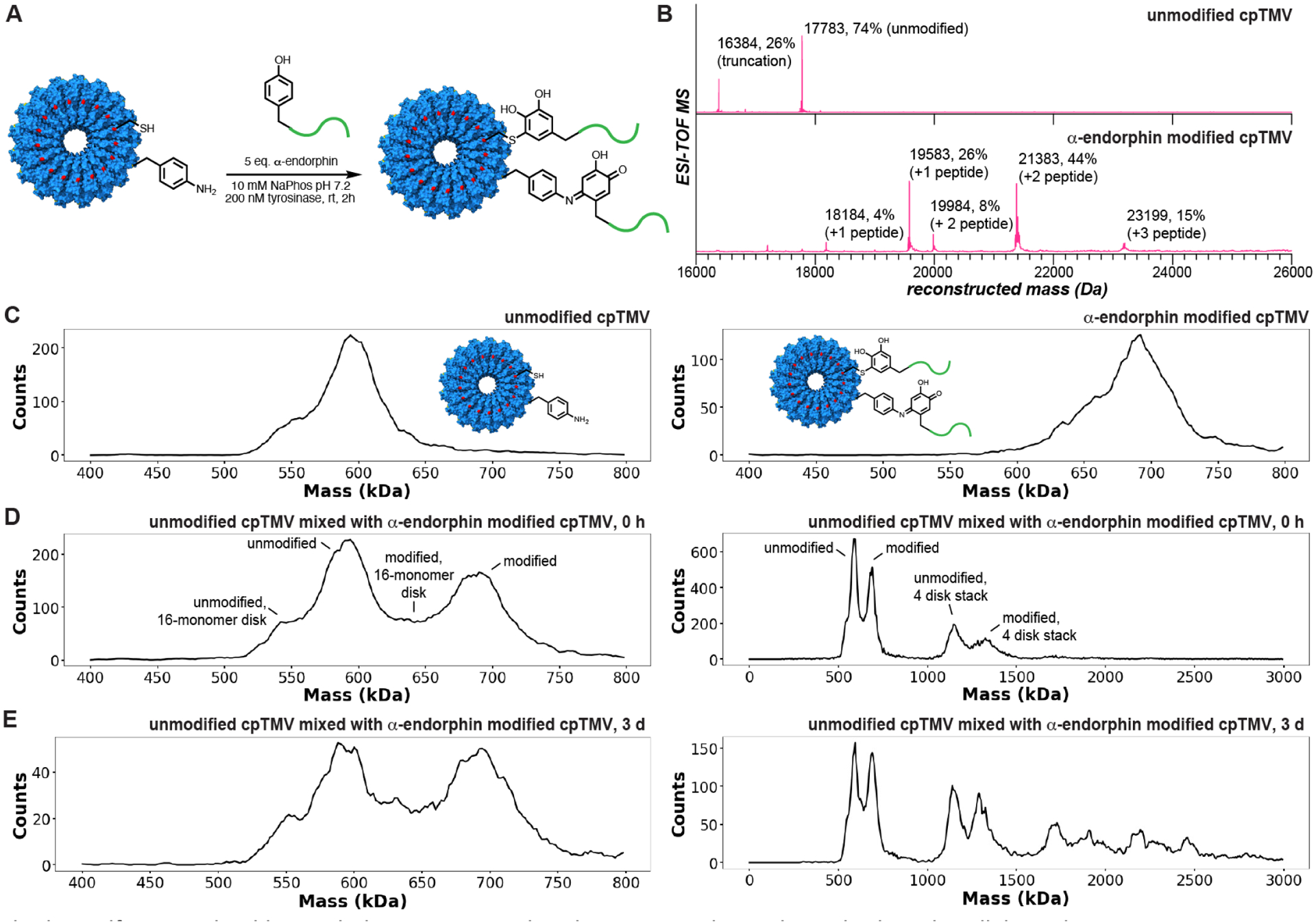
Monitoring protein assembly dynamics using charge detection mass spectrometry. (A) cpTMV-S23C-S65-pAF was modified with α-endorphin, a 2 kDa peptide, using the enzyme tyrosinase from Agaricus bisporus (abTyr). (B) One to two copies of α-endorphin were attached per cpTMV-S23C-S65-pAF monomer as assessed by ESI-TOF mass spectrometry. (C) A comparison of the mass histograms of unmodified and α-endorphin-modified cpTMV-S23C-S65-pAF disks in 100 mM ammonium acetate solution shows a clear difference in size of approximately 90 kDa. A mixture of the unmodified and α-endorphin-modified disks in 100 mM ammonium acetate solution (D) immediately after mixing and (E) incubated at rt 3 days after mixing displays two distinct populations that do not equilibrate over time. For (D) and (E), both a window showing only the two-disk stack and a larger window showing higher stacking stoichiometries are displayed. A statistical analysis of the intact disk distributions based on the monomer % modification is shown in Figure S5.
