Table 1.
Optimization of thioamide synthesis by reacting nitroalkanes with various sulfur sourcesa
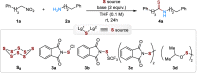 | |||
|---|---|---|---|
| Entry | S source | Base | Yield (%)b |
| 1 | S8 | K2CO3 | 37 |
| 2 | S8 | Na2CO3 | 24 |
| 3 | S8 | Cs2CO3 | 28 |
| 4 | S8 | Na2S | 98 |
| 5 | S8 | Et3N | 16 |
| 6 | S8 | DBU | <5 |
| 7 | S8 | pyridine | <5 |
| 8 | S8 | no base | 12 |
| 9 | 3a | Na2S | 62 |
| 10 | 3b | Na2S | <5 |
| 11 | 3c | Na2S | 50 |
| 12 | 3d | Na2S | 70 |
THF tetrahydrofuran, DBU 1,8-Diazabicyclo[5.4.0]undec-7-ene.
aReaction conditions: Nitroalkane 1a (0.2 mmol), amine 2a (0.4 mmol), S source (0.4 mmol), base (0.4 mmol) in THF (2 mL, 0.1 M).
bIsolated yield.
