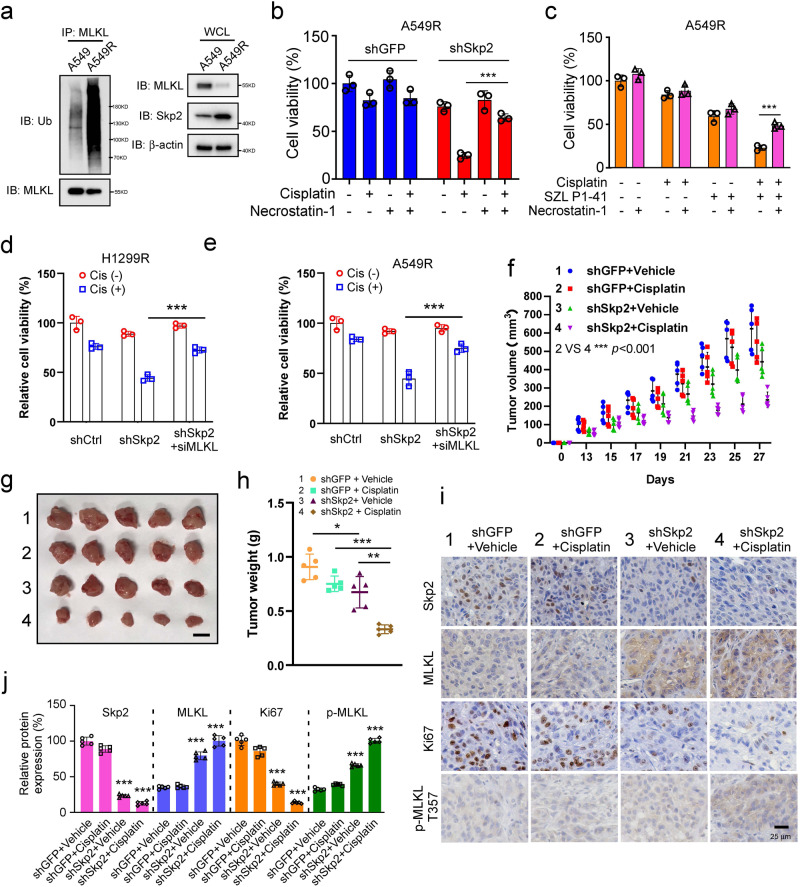
Fig. 7. Skp2-ubiquitinated MLKL degradation confers cisplatin-resistant of NSCLC cells in vivo.
a The parental A549 cells and cisplatin-resistant A549R cells were treated with MG132 for 6 h, WCEs were prepared and subjected to MLKL ubiquitination analysis. b Cisplatin-resistant A549R stable cells were pretreated with 50 μM Necrostatin-1 for 1 h and then treated with DMSO or cisplatin (20 μM) for 48 h. Cell viability was measured. Data represent mean ± SD from three independent experiments. ***p < 0.001 by Student’s t test. c Cisplatin-resistant A549R cells were pretreated with 50 μM Necrostatin-1 for 1 h and then treated with DMSO, cisplatin (20 μM), SZL P1-41 (10 μM) or a cisplatin and SZL P1-41combination for 48 h. Cell viability was measured. Data represent mean ± SD from three independent experiments. ***, p < 0.001 by Student’s t test. d, e H1299R-shSkp2 and A549R-shSkp2 stable cells were transiently knockdown MLKL by siRNA following without/with cisplatin treatment and conducted cell viability assays. Data represent mean ± SD from three independent experiments. **p < 0.01, ***p < 0.001 by Student’s t test, significant difference compared with the cisplatin-treated Skp2-knockdown cells. f–h Tumorigenesis of cisplatin-resistant A549R stable cells treated with vehicle or cisplatin. Tumor volume was recorded (f), tumor size was monitored (g), and tumors were weighed (h). Scale bar, 1 cm. N = 5 mice per group. *p < 0.05, **p < 0.01, ***p < 0.001 1-way ANOVA test with Dunnett’s multiple comparisons test. i, j IHC staining of Skp2, MLKL, Ki67 and phospho-MLKL Thr357 in xenograft tumors from the vehicle or cisplatin-treated shGFP-A549R and shSkp2-A549R stable cells. Data represent mean ± SD from three independent experiments. Scale bar, 25 μm. ***p < 0.001 1-way ANOVA test with Dunnett’s multiple comparisons test.