ABSTRACT.
Early-life experiences of enteric infections and diarrheal illness are common in low-resource settings and are hypothesized to affect child development. However, longer-term associations of enteric infections with school-age cognitive outcomes are difficult to estimate due to lack of long-term studies. The objective of this study was to examine the relationship between enteropathogen exposure in the first 2 years of life with school-age cognitive skills in a cohort of children followed from birth until 6 to 8 years in low-resource settings in Brazil, Tanzania, and South Africa. The study included participants from three sites from the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health Study who were enrolled just after birth and followed for enteric infections, diarrheal illness, and cognitive development until 2 years of age. When the children were school-age, further data were collected on reasoning skills and semantic/phonemic fluency. We estimated associations between the burden of specific enteric pathogens and etiology-specific diarrhea from 0 to 2 years with cognitive test scores at 6 to 8 years using linear regression and adjusting for confounding variables. In this study, children who carried more enteric pathogens in the first 2 years of life showed overall decreases in school-age cognitive abilities, particularly children who carried protozoa, although this was not statistically significant in this sample. Socioeconomic factors such as maternal education and income were more closely associated with school-age cognitive abilities. Early-life enteric pathogens may have a small, lasting influence on school-age cognitive outcomes, although other socioeconomic factors likely contribute more significantly.
INTRODUCTION
Enteric infections are common among children in low- and middle-income countries.1 In addition to causing diarrheal illness, enteric infections contribute to environmental enteropathy and have been associated with malnutrition, growth shortfalls, school days lost due to poor health, and challenges to developmental progress in children.2,3 Importantly, even in the absence of diarrhea, high burdens of enteric pathogens may have impacts on children as they grow.4,5 For example, intestinal bacterial burden in nondiarrheal stools and burden of specific pathogens, including Shigella, Campylobacter, Giardia, and enteroaggregative Escherichia coli, negatively correlated with anthropometric measures at 2 and 5 years of age in the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health (MAL-ED) birth cohort study.5,6
Although early-life anthropometric measures are associated with cognitive outcomes7 and effects on anthropometry are often assumed to correspond to effects on cognition, few studies are able to follow children from birth through school years to assess associations directly between early-life exposures and cognitive/academic outcomes. School-age performance is an important predictor of future independence and contributions to communities.
The MAL-ED is a prospective cohort study that aims to understand the interconnected relationship between malnutrition and enteric disease, and how they affect the development of children in low-resource settings. Children from the MAL-ED Study were found to have increased fecal biomarkers for intestinal inflammation, systemic inflammation, and/or intestinal permeability.8,9 In addition, the burden of enteropathogens and days with illness negatively correlated with cognitive outcomes measured at the age of 2 in MAL-ED.10 However, the long-term impact of early-life infections on cognitive development is unknown. In this study, we measured reasoning skills and verbal fluency in children in three of the MAL-ED sites at age 6-8 years to assess whether enteric disease early in life and socioeconomic status were associated with school-aged cognitive outcomes.
MATERIALS AND METHODS
The overall study design of the MAL-ED study,11 participant baseline characteristics,12–14 and primary outcomes,15 have been described elsewhere. To summarize, eight sites with high rates of malnutrition and enteric disease were chosen, and children were followed from birth to 24 months of life. Information was collected on enteric pathogens, nutrition, sanitation, socioeconomic status, growth and cognitive outcomes. For this study, in three of the participating sites, further cognitive data were collected when the children were 6 to 8 years of age. In this study, we report the relationship between early enteropathogen infections, diarrheal illnesses, socioeconomic status, and cognitive development of 8-year-old children in the MAL-ED cohort.
Predictors.
Nondiarrheal stool samples were collected monthly from the children enrolled in the study from 1 to 24 months of age. These samples were analyzed for 29 enteric pathogens using quantitative polymerase chain reaction (qPCR), as previously described.16 Total pathogen burden was quantified as the average number of pathogens detected in nondiarrheal stools, as well as the total bacterial, viral, and parasitic infection burden. Burden of specific pathogens was calculated as the percent of nondiarrheal stools positive for each pathogen (including the 13 most common pathogens in nondiarrheal stools: Cryptosporidium, Campylobacter, Giardia, Shigella, enteroaggregative E. coli, typical enteropathogenic E. coli (EPEC), atypical EPEC, heat-labile enterotoxigenic E. coli (ETEC), heat-stable (ETEC), norovirus GII, adenovirus 40/41, astrovirus, and sapovirus, as previously described.5 Samples were also collected and tested by qPCR during diarrhea episodes, and diarrhea etiologies were assigned based on the quantity of pathogen detected and the association between pathogens and diarrhea, previously analyzed.17 Briefly, pathogen-attributable fractions were calculated for each episode based on the odds ratios from a mixed effects model associating pathogen quantity with diarrhea. Episodes with pathogen-specific attributable fractions > 0.5 (i.e., majority attribution) were attributed to that pathogen.5 Infectious diarrhea was defined if any pathogen could be attributed to the episode.
Covariates.
In this study, a measure of socioeconomic status was calculated based on the family’s access to clean water, household assets, maternal education, and income.18 We also examined predictors and outcomes by child’s age, gender, research site, maternal education, enrollment weight (proxy for birthweight), maternal height, and breastfeeding status. These variables were measured in infancy.
Outcomes.
The Raven’s Colored Progressive Matrices19,20 (RCPM) is a reasoning assessment developed to assess reasoning skills across the lifespan. In the MAL-ED study, this assessment was used to assess mothers of the participating children when the children were between 6 and 8 months of age.21 The RCPM is a visual, non–language-based assessment that tests the participant’s ability to solve a series of puzzles, set up in analogy form, to form comparisons. The test uses shapes and designs rather than language or pictures, and thus it is thought to be less culture or language specific. The items become progressively more challenging, allowing for assessment of reasoning along a continuum of puzzles. This assessment has been used before in populations that may not have access to formal schooling.20 Because the research sites have experience with this measure, and it remained appropriate for children who may not have attended school and is language-independent, we chose to use the RCPM in the 6- to 8-year-old children as well. The assessment was piloted in all three sites on children not participating in the MAL-ED study and found to be acceptable to the children and provide a variety of scores. The assessment was then administered to children from the MAL-ED study by trained assessors in as quiet and distraction-free location as available. Children were shown example problems and asked to point to the correct answer. When a child had five incorrect answers in a row, the assessment was stopped.
Next, verbal fluency was assessed in the children, using a brief assessment of a subtest from the NEPSY Neuropsychological Exam.22 Semantic fluency was assessed by asking the children to name as many animals as they could in 1 minute and then to name as many foods as they could in 1 minute. Phonemic fluency was assessed by asking children to name as many words that began with S they could in 1 minute and then to name as many words that begin with F as they could in 1 minute. This assessment was chosen because it is a brief, simple assessment that has been used at our sites in the past. In addition, there is previous evidence that childhood diarrhea burden may have associations with verbal fluency.23 This assessment was piloted on children outside the study in all three sites using foods, animals, and words that start with S, N, F, and P. In all languages, children generated more words beginning with S than F (indicating progressing difficulty) and there were relevant words in all languages. z-scores were created for each score, by site, to allow for comparison of child’s performance relative to peers from their site, across sites. One z-score is a single standard deviation difference based on the standard deviation of scores observed in our study population.
Analysis.
Variables were examined and assessed for normality. First, we estimated the associations between total pathogen burden scores in nondiarrheal stool samples for all pathogens, bacteria, viruses, and protozoa with semantic fluency, phonemic fluency, and reasoning skills scores using linear regression, and adjusting for site, sex, maternal education, enrollment weight-for-age z-score, percent days exclusively breastfed in the first 6 months of life, maternal height, socioeconomic status, and age at cognitive outcome assessment. Effect sizes are interpreted per additional pathogen detected on average in nondiarrheal stools from 1 to 24 months of age. Next, the associations between then burden of specific pathogens in nondiarrheal stools and the three cognitive outcomes were estimated using similar models that were additionally adjusted for the other pathogens. Effects were scaled to compare a high burden of pathogen (at the site-specific 90th percentile of stool positivity between 1 and 24 months) to a low burden of pathogen (at the 10th percentile of stool positivity between 1 and 24 months), as previously.5 Scores were reported as change in z-score, with 95% CIs and statistical significance (P values) reported as well.
We next estimated the associations among all-cause, infectious, class-specific, and etiology-specific diarrhea and the cognitive outcomes using linear regression and adjusting for the same covariates listed earlier. Associations with all-cause and infectious diarrhea episodes are scaled per additional episode experienced from birth to 2 years of age. Because few children had multiple etiology-specific episodes, for specific etiologies, we compared children who had at least one episode to children who had no episodes attributed to that pathogen. Finally, we estimated the associations between baseline sociodemographic characteristics and the three cognitive outcomes using multivariable linear regression.
RESULTS
Table 1 shows demographics of the study population in total and by study site. In general, children at the study site in Fortaleza, Brazil were assessed at a younger age than the children in the Venda, South Africa and Haydom, Tanzania (TZH) research sites and thus age was included as a variable in all statistical models. Socioeconomic status (measured through access to clean water, family assets, maternal education, and family income) was generally lowest in the TZH site.
Table 1.
Study population characteristics
| Parameter | Median (IQR) | |||
|---|---|---|---|---|
| Fortaleza, Brazil (N = 117) | Venda, South Africa (N = 171) | Haydom, Tanzania (N = 163) | All (N = 451) | |
| Sociodemographic characteristics | ||||
| Age at 6- to 8-year assessment | 6.85 (6.44 to 7.24) | 8.01 (7.05 to 8.16) | 7.70 (7.33 to 8.02) | 7.49 (7.04 to 8.02) |
| Age at 24-month assessment | 2.03 (2.0 to 2.04) | 2.01 (1.98 to 2.03) | 2.01 (1.97 to 2.16) | 2.02 (1.99 to 2.04) |
| Female sex, n (%) | 53 (45.3) | 84 (49.12) | 82 (50.31) | 219 (48.56) |
| Male sex, n (%) | 64 (54.7) | 87 (50.88) | 81 (49.69) | 232 (51.44) |
| % days exclusively breastfed < 6 months | 0.49 (0.28 to 0.71) | 0.17 (0.1 to 0.29) | 0.32 (0.19 to 0.44) | 0.28 (0.15 to 0.46) |
| Mean WAMI* score | 0.83 (0.77 to 0.90) | 0.8 (0.72 to 0.85) | 0.21 (0.14 to 0.28) | 0.72 (0.27 to 0.84) |
| Maternal height (cm) | 155 (150 to 160) | 158 (155 to 162) | 156.5 (152 to 160) | 157 (152 to 161) |
| Maternal education (years) | 9 (7 to 12) | 11 (9 to 12) | 7 (3 to 7) | 8 (7 to 11) |
| Improved water, n (%) | 117 (100) | 157 (92.35) | 103 (63.19) | 377 (83.78) |
| Improved sanitation, n (%) | 117 (100) | 167 (98.24) | 10 (6.1) | 294 (65.33) |
| Monthly income (U.S. dollars) | 345.81 (292.13 to 412.81) | 246.51 (164.37 to 396.84) | 21.0 (13.17 to 39.0) | 186 (32.56 to 344.93) |
| No. of diarrheal episodes 0–24 months | 1 (0 to 2) | 1 (0 to 2) | 2 (1 to 4) | 1 (0 to 2) |
| Anthropometry | ||||
| Enrollment WAZ | −0.17 (−0.77 to 0.47) | −0.40 (−0.96 to 0.14) | −0.02 (−0.61 to 0.64) | −0.20 (−0.83 to 0.43) |
| 2-year LAZ | −0.03 (−0.87 to 0.73) | −1.62 (−2.46 to −1.05) | −2.59 (−3.22 to −1.98) | −1.73 (−2.60 to −0.74) |
| 5-year HAZ | −0.08 (−0.76 to 0.38) | −1.05 (−1.56 to −0.40) | −1.93 (−2.53 to −1.24) | −1.15 (−1.92 to −0.36) |
| 6- to 8-year HAZ | 0.13 (−0.74 to 0.54) | 0.14 (−0.82 to 0.52) | −1.56 (−2.15 to −1.03) | −0.65 (−1.56 to 0.15) |
HAZ = height-for-age z-score; IQR = interquartile range; LAZ = weight-for-age z-score; WAMI = Water/Sanitation, Assets, Maternal education, and Income; WAZ = weight-for-age z-score.
WAMI is a measure of socioeconomic status that includes family’s access to clean water, household assets, maternal education, and income.
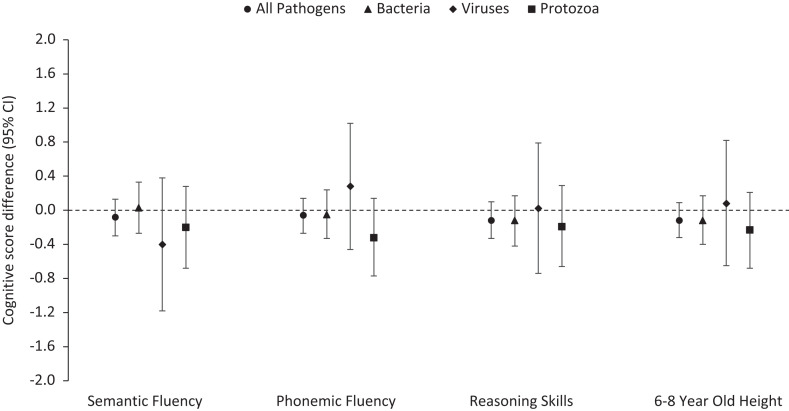
In general, larger enteric pathogen burdens were associated with lower cognitive scores (Figure 1), even when controlling for site, breastfeeding status, sex, enrollment weight, socioeconomic status, maternal height, maternal education, and age, although the associations were not statistically significant. For example, an additional enteric pathogen detected on average in nondiarrheal stools in the first 2 years of life was associated with −0.12 z-scores (95% CI: −0.33 to 0.10) on the Raven assessment. Negative associations were observed for bacterial and protozoal pathogens but not viral pathogens. A higher burden of protozoa in nondiarrheal stool samples had the strongest association with reasoning skills (Raven z-score difference per additional protozoa detected on average in nondiarrheal stool samples: −0.19, 95% CI: −0.66 to 0.29) and semantic (z-score difference: −0.20, 95% CI: −0.68 to 0.28) and phonemic fluency (z-score difference: −0.32, 95% CI: −0.77 to 0.14). Similar negative associations were observed between enteric pathogens before 2 years of age and height-for-age z-score measured at 6 to 8 years.
Figure 1.
Associations between early enteric pathogen infections and cognitive outcomes and growth at school-age. Effect of one additional pathogen detected on average from 0 to 24 months of age. Adjusted for percent exclusive breastfed, age, enrollment weight (birthweight proxy), socioeconomic status, maternal height, maternal education, sex, and research site.
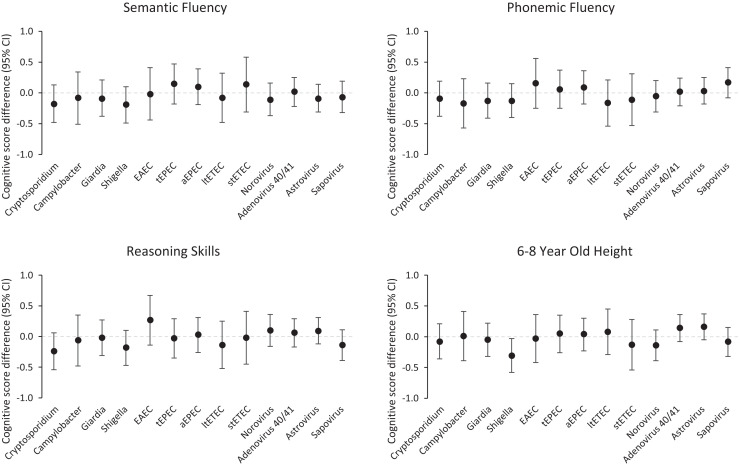
Associations between the burden of specific enteric pathogens in the first 2 years of life and cognitive outcomes at school age were also generally small and not statistically significant (Figure 2). A high burden of Cryptosporidum and Shigella were associated with the largest decrements in cognitive skills. For example, a high burden of Cryptosporidium was associated with −0.24 Raven z-scores (95% CI: −0.54 to 0.06) compared with a low burden of Cryptosporidium. A high burden of Shigella was associated with a −0.18 z-score decrement (95% CI: −0.47 to 0.10) in semantic fluency. In a sensitivity analysis in which pathogen burden was defined by detections in both diarrheal and nondiarrheal stools, the results were unchanged.
Figure 2.
Associations between early specific enteric pathogen infections and cognitive outcomes at school-age. All models adjusted for percent exclusive breastfed, age, enrollment weight (birthweight proxy), socioeconomic status, maternal height, maternal education, sex, and research site.
The associations between etiology-specific diarrhea and 6- to 8-year-old cognitive outcomes were also relatively small (Table 2). In general, additional diarrheal episodes attributed to protozoal pathogens were associated with decrements in cognitive scores, although these estimates were imprecise (z-score difference per additional episode: −1.00, 95% CI: −2.39 to 0.39 for semantic fluency, −1.02, 95% CI: −2.34 to 0.30 for phonemic fluency, −1.11, 95% CI −2.47 to 0.26 for reasoning skills). Conversely, viral diarrhea episodes were more often associated with a positive increase in cognitive skills, although these estimates were not statistically significant (Table 2). Diarrhea episodes attributed to Campylobacter jejuni/coli and adenovirus 40/41 had the strongest negative associations with semantic and phonemic fluency. Norovirus GII and sapovirus diarrhea episodes had the strongest negative association with reasoning skills. In contrast to the associations with subclinical Shigella infections, Shigella-attributable diarrhea episodes were not associated with semantic fluency or reasoning skills and had a small negative association with phonemic fluency (z-score difference: −0.15, 95% CI: −0.54 to 0.23).
Table 2.
Associations between etiology-specific diarrhea and school-age cognitive outcomes
| Parameter | Estimate (CL) P value | |||
|---|---|---|---|---|
| Semantic fluency | Phonemic fluency | Reasoning skills | Height | |
| Words/animals in a minute from NEPSY | Words beginning with S & F in a minute from NEPSY | Raven colored progressive matrices | Measured during school-aged testing | |
| Number of diarrhea episodes in 0–24 months | ||||
| Any episodes* | 0.005 (−0.05, 0.06) 0.86 | −0.001 (−0.05, 0.05) 0.96 | −0.04 (−0.10, 0.01) 0.12 | 0.003 (−0.05, 0.06) 0.92 |
| Any episodes attributable to a specific pathogen* | 0.08 (−0.08, 0.23) 0.33 | 0.01 (−0.13, 0.16) 0.85 | −0.06 (−0.20, 0.09) 0.46 | 0.03 (−0.12, 0.18) 0.70 |
| Episodes attributable to bacteria* | −0.02 (−0.29, 0.26) 0.91 | −0.06 (−0.32, 0.20) 0.66 | 0.09 (−0.18, 0.36) 0.50 | 0.07 (−0.20, 0.34) 0.61 |
| Episodes attributable to viruses* | 0.20 (−0.06, 0.46) 0.12 | 0.05 (−0.19, 0.30) 0.68 | −0.25 (−0.50, 0.004) 0.05 | −0.07 (−0.34, 0.19) 0.58 |
| Episodes attributable to protozoa* | −1.00 (−2.39, 0.39) 0.16 | −1.02 (−2.34, 0.30) 0.13 | −1.11 (−2.47, 0.26) 0.11 | −0.34 (−1.66, 0.99) 0.62 |
| Comparison of children with ≥ 1 episode of diarrhea attributable to a specific pathogen with children with 0 episodes attributable to that pathogen | ||||
| Campylobacter jejuni/coli | −0.60 (−1.38, 0.17) 0.13 | −0.23 (−0.97, 0.52) 0.55 | 0.17 (−0.56, 0.89) 0.65 | −0.11 (−0.91, 0.69) 0.79 |
| Sapovirus | 0.52 (0.09, 0.97) 0.02 | 0.38 (−0.04, 0.80) 0.08 | −0.19 (−0.63, 0.25) 0.40 | −0.11 (−0.54, 0.33) 0.63 |
| Enterotoxigenic E. coli | −0.26 (−0.70, 0.11) 0.16 | 0.09 (−0.30, 0.48) 0.64 | 0.05 (−0.46, 0.35) 0.80 | 0.11 (−0.30, 0.51) 0.61 |
| Astrovirus | 0.09 (−0.45, 0.63) 0.75 | 0.23 (−0.30, 0.75) 0.39 | −0.13 (−0.67, 0.41) 0.64 | −0.06 (−0.63, 0.51) 0.85 |
| Norovirus GII | 0.18 (−0.22, 0.62) 0.35 | −0.04 (−0.45, 0.36) 0.83 | −0.21 (−0.63, 0.21) 0.33 | 0.0004 (−0.43, 0.43) 1.00 |
| Rotavirus | 0.59 (0.12, 1.06) 0.01 | 0.16 (−0.29, 0.62) 0.48 | 0.04 (−0.43, 0.51) 0.85 | −0.08 (−0.54, 0.39) 0.74 |
| Adenovirus 40/41 | −0.25 (−0.85, 0.35) 0.42 | −0.37 (−0.95, 0.20) 0.20 | −0.01 (−0.59, 0.57) 0.97 | 0.48 (−0.09, 1.06) 0.10 |
| Shigella | −0.02 (−0.42, 0.39) 0.93 | −0.15 (−0.54, 0.23) 0.44 | 0.04 (−0.36, 0.43) 0.86 | 0.09 (−0.31, 0.49) 0.66 |
CL = confidence limits. Adjusted for %exclusive breastfed, age, enrolment weight (birthweight proxy), socioeconomic status, maternal height, maternal education, sex and research site.
Effects per additional episode attributed.
Compared with the associations between enteric pathogens and the school-age cognitive outcomes, sociodemographic variables were more strongly associated with the cognitive outcomes (Table 3). Maternal height and maternal education were positively related to semantic fluency and reasoning skills. Family income was significantly associated with all the cognitive outcomes measured. Height measurements at 2, 5, and 6 to 8 years of age were strongly associated with the cognitive outcomes. For example, a 1 unit increase in length-for-age z-score at age 2 years was associated with 0.17 z-score increase (95% CI: 0.08–0.27) in semantic fluency, a 0.17 z-score increase (95% CI: 0.08–0.26) in phonemic fluency, and a 0.14 z-score increase (95% CI: 0.05–0.24) in reasoning skills. Associations were slightly stronger between height at 6 to 8 years and cognition at the same age.
Table 3.
Childhood factors associated with semantic fluency and reasoning
| Parameter | Estimate (CL) P value | ||
|---|---|---|---|
| Semantic fluency | Phonemic fluency | Reasoning skills | |
| Words/animals in a minute from NEPSY | Words beginning with S & F in a minute from NEPSY | Raven colored progressive matrices | |
| Age at assessment | 0.002 (−0.19, 0.19) 0.98 | 0.43 (0.24, 0.62) <0.0001 | 0.14 (−0.05, 0.33) 0.14 |
| Enrollment weight (proxy for birthweight) | 0.04 (−0.06, 0.14) 0.41 | 0.13 (0.04, 0.23) 0.01 | 0.09 (−0.01, 0.19) 0.06 |
| Maternal height | 0.01 (−0.003, 0.03) 0.12 | 0.02 (0.01, 0.04) 0.006 | 0.02 (0.002, 0.03) 0.03 |
| Maternal education | 0.03 (−0.01, 0.06) 0.11 | 0.05 (0.02, 0.09) 0.003 | 0.04 (0.01, 0.08) 0.02 |
| WAMI (socioeconomic status) | 1.13 (0.24, 2.03) 0.01 | 1.70 (0.81, 2.55) 0.0002 | 1.20 (0.31, 2.09) 0.01 |
| Income (/$100) | 0.08 (0.03, 0.12) 0.001 | 0.07 (0.02, 0.11) 0.004 | 0.05 (0.01, 0.10) 0.02 |
| Percent days exclusively breastfed | 0.07 (−0.40, 0.54) 0.76 | 0.41 (−0.05, 0.87) 0.08 | −0.17 (−0.63, 0.28) 0.46 |
| Sex (female versus male) | 0.10 (−0.09, 0.29) 0.30 | −0.23 (−0.41, −0.05) 0.01 | 0.08 (−0.1, 0.27) 0.39 |
| 2 year-old height | 0.17 (0.08, 0.27) 0.0003 | 0.17 (0.08, 0.26) 0.0002 | 0.14 (0.05, 0.24) 0.002 |
| 5 year-old height | 0.19 (0.09, 0.29) 0.0002 | 0.20 (0.1, 0.30) <0.0001 | 0.21 (0.11, 0.31) <0.0001 |
| Height at assessment (∼8 year-old height) | 0.22 (0.12, 0.31) <0.0001 | 0.27 (0.17, 0.36) <0.0001 | 0.20 (0.11, 0.29) <0.0001 |
CL = confidence limits; WAMI = Water/Sanitation, Assets, Maternal education, and Income. Models adjusted for study site and age.
DISCUSSION
This study assessed longer-term follow-up cognitive testing at school age in a longitudinal birth cohort to examine relationships between early-life enteric pathogen exposures and school-age cognitive outcomes. Bacterial and protozoal pathogens showed small negative associations with cognitive outcomes, although the estimates were imprecise. The research sample may not be large enough to detect small changes; however, even when controlling for many variables that are associated with school-age outcomes, these results are consistent with prior work on the impact of early-life enteric infections on longer-term morbidity. For example, we found that bacterial and protozoal infections were associated with linear growth decrements at 2 and 5 years of age in MAL-ED, but viral infections were not.5 Bacterial and protozoal pathogens may have larger effects on the gut than viruses and contribute to environmental enteric dysfunction, leading to long-term impacts on growth and cognition. Specifically, Shigella and Campylobacter are inflammatory pathogens, and both intestinal and systemic inflammation may be associated with poorer cognitive function.
It is potentially interesting that early-life Cryptosporidium and Shigella infections tended to associate with the lowest semantic fluency and reasoning (Figure 2). In a companion paper focused on Shigella infections and inflammation24, we demonstrate that the association of early-life Shigella prevalence with lower height-for-age-z-score at school age is consistent across the three sites and estimate site-specific associations of Shigella burdens with modest but consistently lower cognitive outcomes (reasoning and semantic and verbal fluency) in Brazil and Tanzania, but not South Africa. Hence, further work with rapid, simple fecal diagnostics for selected pathogens or inflammation that could be done in field settings may enable research to focus on subgroups in certain settings that could benefit from targeted antimicrobial and nutritional therapy.
This study had the benefit of longitudinal follow-up of children from birth to 6–8 years of age, as well as frequent monthly collection of stools to characterize enteric pathogen burden in the first 24 months of life in three settings of malnutrition and enteric disease. One limitation of this data is that by 6 to 8 years of age, many of the children in the research site in Tanzania still had not had much formal schooling, and thus the second executive function task of naming words that begin with certain letters was more challenging for them. Children in all three sites in settings of malnutrition, enteric disease, and low resources were more likely to be below age expectancy for these cognitive assessments.
Our study relied on the lessons from the previous work of the cognitive assessment team of the MAL-ED study.21 We found that it is difficult to measure cognitive development across languages and cultures. However, by using assessments that are less reliant on specific culture and language and that have been piloted with children in the area, a distribution of results can be obtained. This study only measured three areas of childhood cognitive abilities: semantic fluency, phonemic fluency, and reasoning skills. Although these are important areas of functioning, other studies may measure other important areas of childhood cognition to continue to examine the role of early enteric pathogen exposure in child development.
There are many proximal factors to school-age cognition and learning, and the ones that are likely most influential early in life, such as maternal education level and family resources, were found to have stronger associations with higher cognitive scores in this study. These results suggest that interventions directly on cognitive development, such as cognitive stimulation, may have larger impacts on cognitive development. Child growth was also associated closely with cognitive scores, highlighting the importance of nutrition. However, there may also be a small role of interventions for early-life enteric pathogens to improve cognitive abilities in school-age children.
ACKNOWLEDGMENTS
We thank the children and families who participated in the MAL-ED study, as well as the field teams and all MAL-ED contributors.
REFERENCES
- 1. Troeger C. et al. , 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18: 1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mata LJ, 1978. The Children of Santa Maria Cauque: A Prospective Field Study of Health and Growth. Cambridge, MA: MIT Press. [Google Scholar]
- 3. Petri WA, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL, 2008. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest 118: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeBoer MD, Lima AA, Oría RB, Scharf RJ, Moore SR, Luna MA, Guerrant RL, 2012. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr Rev 70: 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogawski ET. et al. , 2018. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health 6: e1319–e1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richard SA. et al. , 2019. Enteric dysfunction and other factors associated with attained size at 5 years: MAL-ED birth cohort study findings. Am J Clin Nutr 110: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scharf RJ. et al. , 2018. Early childhood growth and cognitive outcomes: findings from the MAL-ED study. Matern Child Nutr 14: e12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kosek M. et al. , 2013. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg 88: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Investigators M-EN , 2017. Relationship between growth and illness, enteropathogens and dietary intakes in the first 2 years of life: findings from the MAL-ED birth cohort study. BMJ Glob Health 2: e000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Acosta AM. et al. , 2018. Early childhood cognitive development is affected by interactions among illness, diet, enteropathogens and the home environment: findings from the MAL-ED birth cohort study. BMJ Glob Health 3: e000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MAL-ED-Network-Investigators , 2014. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis 59: S193–S206. [DOI] [PubMed] [Google Scholar]
- 12. Lima AA. et al. , 2014. Geography, population, demography, socioeconomic, anthropometry, and environmental status in the MAL-ED cohort and case-control study sites in Fortaleza, Ceará, Brazil. Clin Infect Dis 59 (Suppl 4): S287–S294. [DOI] [PubMed] [Google Scholar]
- 13. Mduma ER. et al. , 2014. The etiology, risk factors, and interactions of enteric infections and malnutrition and the consequences for child health and development study (MAL-ED): description of the Tanzanian site. Clin Infect Dis 59: S325–S330. [DOI] [PubMed] [Google Scholar]
- 14. Bessong PO, Nyathi E, Mahopo TC, Netshandama V, Africa M-ES, 2014. Development of the Dzimauli community in Vhembe District, Limpopo Province of South Africa, for the MAL-ED cohort study. Clin Infect Dis 59: S317–S324. [DOI] [PubMed] [Google Scholar]
- 15. MAL-ED_Network_Investigators , 2017. Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: the MAL-ED longitudinal birth cohort study. PLoS Med 14: e1002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Platts-Mills JA, McCormick BJ, Kosek M, Pan WK, Checkley W, Houpt ER; MAL-ED Network Investigators , 2014. Methods of analysis of enteropathogen infection in the MAL-ED cohort study. Clin Infect Dis 59 (Suppl 4): S233–S238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Platts-Mills JA. et al. , 2018. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 6: e1309–e1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Psaki SR. et al. , 2014. Measuring socioeconomic status in multicountry studies: results from the eight-country MAL-ED study. Popul Health Metr 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bingham WC, Burke HR, Murray S, 1966. Raven’s progressive matrices: construct validity. J Psychol 62: 205–209. [DOI] [PubMed] [Google Scholar]
- 20. Raven J, 2000. The Raven’s progressive matrices: change and stability over culture and time. Cognit Psychol 41: 1–48. [DOI] [PubMed] [Google Scholar]
- 21. Murray-Kolb LE. et al. , 2014. The MAL-ED cohort study: methods and lessons learned when assessing early child development and caregiving mediators in infants and young children in 8 low- and middle-income countries. Clin Infect Dis 59 (Suppl 4): S261–S272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korkman M, 1987. NEPSY – a neuropsychological test battery for children, based on Luria’s investigation. J Clin Exp Neuropsychol 9: 268. [Google Scholar]
- 23. Patrick PD, Oriá RB, Madhavan V, Pinkerton RC, Lorntz B, Lima AA, Guerrant RL, 2005. Limitations in verbal fluency following heavy burdens of early childhood diarrhea in Brazilian shantytown children. Child Neuropsychol 11: 233–244. [DOI] [PubMed] [Google Scholar]
- 24. McQuade ETR. et al. , 2022. Impact of Shigella infections and inflammation early in life on child growth and school-aged cognitive outcomes: Findings from three birth cohorts over eight years. PLoS Negl Trop Dis 16: e0010722. [DOI] [PMC free article] [PubMed] [Google Scholar]