Abstract
BACKGROUND:
Dabigatran is a direct thrombin inhibitor approved by the FDA in October 2010 for the treatment of nonvalvular atrial fibrillation. Little is known regarding patient adherence to this therapy.
OBJECTIVE:
To examine adherence and persistence to dabigatran among adults with atrial fibrillation.
METHODS:
We used IMS Health’s LifeLink Health Plan Claims Database from 2010 to 2012 to identify patients with atrial fibrillation who were new users of dabigatran. We derived adherence and persistence for continuously enrolled patients at 6 months, 9 months, and 12 months of follow-up. We measured adherence using the medication possession ratio (MPR), defined as individuals with MPRs of 0.80 or greater as adherent, and examined persistence by identifying individuals with gaps in drug possession of 60 days or greater.
RESULTS:
Of 5,951 adults with atrial fibrillation who were new users of dabigatran, 49% had prevalent atrial fibrillation and at least 6 months of continuous follow-up. Of these, 89% used dabigatran as the only oral anticoagulant, whereas the remainder filled prescriptions for at least 1 other oral anticoagulant during the follow-up period. Among those using dabigatran alone (n = 2,713), the mean MPR was 0.73 (standard error = 0.30), 41% were nonadherent with therapy, and 32% had gaps of 60 days or greater. Among those observed for 9 (or 12) months who used dabigatran alone, rates of nonadherence were 47% (49%), whereas 48% (49%) discontinued therapy during follow-up. Rates of adherence and persistence were similar for patients with incident atrial fibrillation.
CONCLUSIONS:
Nonadherence to dabigatran was common among patients with atrial fibrillation. Future studies are needed to understand the reasons for nonadherence.
What is already known about this subject
Dabigatran is a direct thrombin inhibitor approved by the FDA for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
Dabigatran offers a convenient dosing regimen and does not require frequent laboratory monitoring of drug levels.
Adherence rates have been as high as 85%-98% in clinical trials; limited studies have evaluated adherence in the real-world setting.
What this study adds
At 6 months of initiating dabigatran, the mean medication possession ratio (MPR) of prevalent atrial fibrillation patients is 0.73, and 2 in 5 dabigatran users had MPRs < 0.80.
The mean MPR and percentage of nonadherence (MPR < 0.80) decreased as the length of follow-up increased. At 1 year, the average MPR was 0.65, and 49.2% of patients failed to adhere to dabigatran (MPR < 0.80).
Atrial fibrillation, the most common type of cardiac arrhythmia, affects 0.4%-1% of the general population.1 The incidence of atrial fibrillation increases significantly with age.1 Patients with atrial fibrillation have 4 to 5 times elevated risk for ischemic stroke, with higher risk among older adults.2 In October 2010, dabigatran etexilate, a direct thrombin inhibitor, was approved by the U.S. Food and Drug Administration for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.3 This approval was based on the results of the RE-LY trial that demonstrated dabigatran to be noninferior to warfarin (vitamin K antagonist) in reducing stroke or systemic embolism.4 Compared with warfarin, the mainstay anticoagulant therapy for over 50 years, dabigatran offers a more convenient anticoagulant therapy for patients and physicians. Dabigatran is administered at a fixed dose and does not require frequent lab monitoring or dose adjustments in order to avoid adverse events of bleeding or subtherapeutic dosing. However, the absence of any requirement for laboratory monitoring may lead to fewer clinical encounters during which the importance of adherence could be reinforced.5 Moreover, dabigatran is administered twice daily rather than once daily, which could also lead to poorer adherence.6
Less is known about adherence to novel oral anticoagulants, and what evidence that does exist suggests limited nonadherence to dabigatran, the first novel agent to be approved in clinical trials in the United States.7-9 In the RE-LY trial, 15% of atrial fibrillation patients discontinued 150 mg dabigatran at 1 year and 21% discontinued at 2 years.4 The RE-COVER trial, which evaluated dabigatran vs. warfarin for the treatment of acute venous thromboembolism, reported an adherence rate of 98%.10 Adherence in clinical trials is typically higher than in usual practice due to selection of more highly motivated patients at baseline and greater monitoring and follow-up of trial participants.11 We examined adherence and persistence to dabigatran among adults with nonvalvular atrial fibrillation in a real-world setting.
Methods
Data
We used data from the nationally representative IMS Health LifeLink Health Plan Claims Database. This dataset provides information on commercial health plan claims from managed care plans and other sources throughout the United States. The database includes information on inpatient and outpatient diagnoses in the format of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes and procedures in the format of Current Procedural Terminology (CPT-4) and Healthcare Common Procedure Coding System codes. Retail and mail-order prescription records, which include National Drug Code (NDC) numbers and quantity of dispensed medication, are all available. Dates of service for all claims, the charge allowed and paid amount of all claims, patient demographic characteristics (including age, gender, and geographic region where the patient resides), payer type (such as commercial, self-pay), health plan product type (such as health maintenance organization, preferred provider organization), provider specialty, and the start and end date of the enrollment are available.
Study Population
We included patients with prevalent atrial fibrillation who filled at least 1 prescription for dabigatran from October 1, 2010 through March 31, 2012. Patients with prevalent atrial fibrillation were identified as those who had an ICD-9-CM diagnosis code of 427.31 before the first date of filling a prescription for dabigatran (index date). Diagnoses of atrial fibrillation were recorded during the 6-month baseline period prior to the index date. This code had been validated in many studies and showed a positive predictive value (PPV) of 70%-96%.12 Dabigatran use was identified using NDC numbers, which are provided in the Appendix (available in online article).
To be included in the cohort, individuals had to meet the following criteria: (a) aged 18 years and above on the index date; (b) continuous medical and pharmacy enrollment with benefits of 6 months prior to the index date; (c) no prescription claims of anticoagulants, including warfarin, dabigatran, or rivaroxaban, in the previous 6 months prior to the index date (new users); and (d) a prescription of dabigatran between October 1, 2010, and March 31, 2012. To be eligible for each specific follow-up cohort, patients had to have continuous full medical and pharmacy enrollment during the entire follow-up period. Patients who lacked full medical and pharmacy enrollment over the study period were excluded. The end date of the study was either the last date of the follow-up period, withdrawal, death, or the end of the study.
We restricted our primary analysis to individuals whose index date was after the date of diagnosis of atrial fibrillation; that is, prevalent atrial fibrillation patients who were only exposed to dabigatran during the entire follow-up period (nonswitchers). To compare adherence and persistence among patients with incident atrial fibrillation, we also identified incident cohorts by including those with a first diagnosis of atrial fibrillation after the index date in the secondary analysis. We identified the first date of diagnosis of atrial fibrillation for all subjects. Subjects eligible for the incident atrial fibrillation cohort had a diagnosis of atrial fibrillation after the index date and thus no diagnosis for atrial fibrillation over the 6-month baseline period. Furthermore, we identified those individuals who switched from dabigatran to warfarin or rivaroxaban during the follow-up period.
Study Measures and Analysis
Multiple time points were selected to quantify adherence and persistence over time. We derived 3 continuously enrolled patient cohorts with follow-up periods of 6 months, 9 months, and 12 months. Subjects included in the cohorts with longer follow-up periods were subsets of patients in the cohorts with shorter periods. We assessed patients’ demographic characteristics prior to the index date, including age, gender, and region of residence. We calculated the CHADS2 score, a validated clinical classification scheme used to predict the risk of stroke in atrial fibrillation patients by factoring in congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, and prior stroke/transient ischemic attack.13 The CHADS2 score was calculated using medical claims over the baseline period. Diagnoses of congestive heart failure, hypertension, diabetes mellitus, stroke, and transient ischemic attack were identified using ICD-9-CM codes.14,15 The ICD-9-CM codes are presented in the Appendix.
We quantified adherence and persistence using pharmacy claims for dabigatran that were filled during the follow-up period. We measured adherence using the medication possession ratio (MPR), which we calculated as the total number of days with dispensed supply of dabigatran divided by the follow-up period (180 days, 270 days, or 365 days). Since patients may stockpile medications at home, overlaps between claims were allowed and were included in the calculation of the total number of days supply of dabigatran. This may result in a value for the MPR that is greater than 1; we capped the MPR at 1. We excluded any drug supply that covered the post-follow-up time from the analysis. We used a cutoff point of 0.80 to dichotomize individuals as adherent (MPR ≥ 0.80) or nonadherent (MPR < 0.80) as is standard in studies of adherence.16
We characterized persistence by treatment discontinuation, defining individuals with gaps of 60 days or longer in dabigatran supply as failing to persist on their therapy. Sensitivity analysis using gaps of 15 days, 30 days, and 90 days were performed. Among patients who were nonpersistent with dabigatran, we calculated their time to discontinuation. For patients who were switched to a second anticoagulant (warfarin or rivaroxaban) from dabigatran during the follow-up period, we calculated the incidence of switching and time to switch to a second anticoagulant and their adherence to oral anticoagulants (including warfarin, dabigatran, or rivaroxaban). We used the statistical software SAS, version 9.3 (SAS Institute, Inc., Cary, NC) for all analyses, except the Kaplan-Meier curve was plotted using STATA, version 12.1 (Stata Corp, College Station, TX).
Results
Study Population
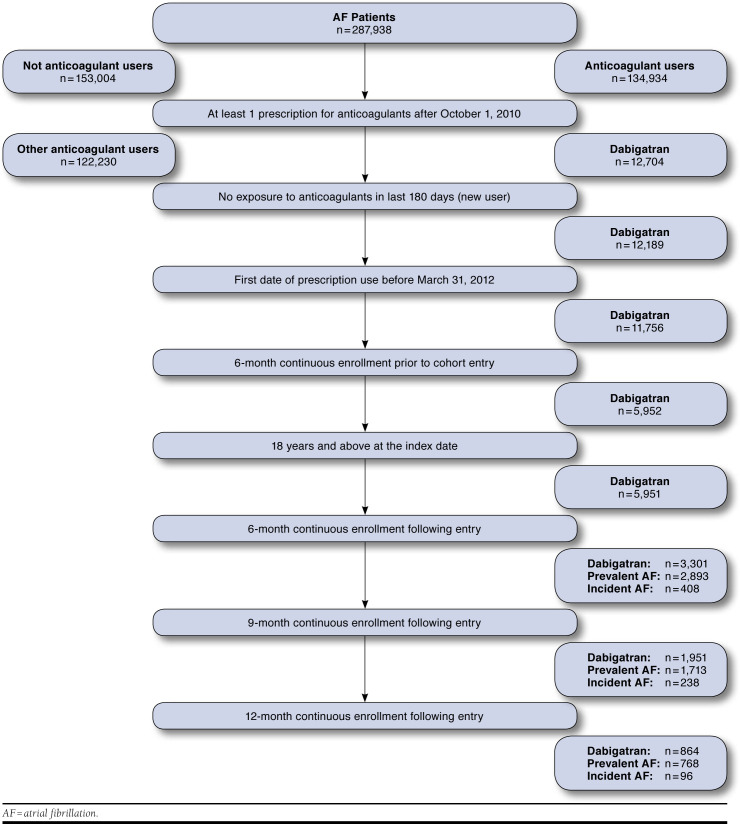
We identified 287,938 patients with nonvalvular atrial fibrillation, of whom 5,951 fulfilled our inclusion criteria for a dabigatran user and were potentially eligible for inclusion in the study. Figure 1 describes the process of sample selection. After we applied the inclusion criteria for continuous enrollment over the follow-up period and then separated prevalent and incident atrial fibrillation patients, we included 2,893 individuals with prevalent atrial fibrillation in the 6-month follow-up cohort; 1,713 individuals in the 9-month cohort; and 768 individuals in the 12-month follow-up cohort. Baseline characteristics of the prevalent cohorts are shown in Table 1. The average age of the study population was 63.0 years across all 3 cohorts and approximately two thirds to three fourths of patients were male.
FIGURE 1.
Sample Selection
TABLE 1.
Baseline Characteristics of Study Population
| Prevalent Atrial Fibrillation | Incident Atrial Fibrillation | |||||
|---|---|---|---|---|---|---|
| Follow-up Period | 6 Months | 9 Months | 12 Months | 6 Months | 9 Months | 12 Months |
| Number of patients | 2,893 | 1,713 | 768 | 408 | 238 | 96 |
| Age, mean (SE) | 63.0 (11.6) | 63.0 (11.6) | 63.0 (11.6) | 64.4 (11.6) | 65.2 (12.3) | 65.0 (12.5) |
| Age, n (%) | ||||||
| 18-44 | 116 (4.0) | 66 (3.9) | 32 (4.2) | 16 (3.9) | 9 (3.8) | 4 (4.2) |
| 45-64 | 1,733 (59.9) | 1,034 (60.4) | 469 (61.1) | 217 (53.2) | 126 (52.9) | 52 (54.2) |
| 65-74 | 552 (19.1) | 320 (18.7) | 141 (18.4) | 97 (23.8) | 51 (21.4) | 17 (17.7) |
| 75+ | 492 (17.0) | 293 (17.1) | 126 (16.4) | 78 (19.1) | 52 (21.8) | 23 (24.0) |
| Gender, n (%) | ||||||
| Male | 1,999 (69.1) | 1,211 (70.7) | 554 (72.1) | 309 (75.7) | 176 (73.9) | 65 (67.7) |
| Female | 894 (30.9) | 502 (29.3) | 214 (27.9) | 99 (24.3) | 62 (26.1) | 31 (32.3) |
| Region of residence, n (%) | ||||||
| East | 295 (10.2) | 164 (9.6) | 88 (11.5) | 38 (9.3) | 25 (10.5) | 11 (11.5) |
| Midwest | 901 (31.1) | 557 (32.5) | 274 (35.7) | 129 (31.6) | 73 (30.7) | 33 (34.4) |
| South | 1,394 (48.2) | 806 (47.1) | 315 (41.0) | 201 (49.3) | 116 (48.7) | 46 (47.9) |
| West | 303 (10.5) | 186 (10.9) | 91 (11.8) | 40 (9.8) | 24 (10.1) | 6 (6.3) |
| CHADS2, mean (SE) | 1.47 (1.13) | 1.44 (1.09) | 1.43 (1.08) | 1.53 (1.12) | 1.52 (1.05) | 1.69 (1.05) |
| CHADS2, n (%) | ||||||
| 0 | 557 (19.3) | 332 (19.4) | 145 (18.9) | 77 (18.9) | 41 (17.2) | 12 (12.5) |
| 1 | 1,089 (37.6) | 651 (38.0) | 302 (39.3) | 131 (32.1) | 78 (32.8) | 30 (31.3) |
| 2 | 776 (26.8) | 465 (27.1) | 209 (27.2) | 138 (33.8) | 85 (35.7) | 36 (37.5) |
| 3 | 314 (10.9) | 184 (10.7) | 80 (10.4) | 36 (8.8) | 23 (9.7) | 12 (12.5) |
| 4 | 117 (4.0) | 64 (3.7) | 23 (3.0) | 22 (5.4) | 10 (4.2) | 6 (6.3) |
| 5 | 37 (1.3) | 16 (0.9) | 8 (1.0) | 4 (1.0) | 1 (0.4) | 0 (0.0) |
| 6 | 3 (0.1) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Source: IMS Health LifeLink Health Plan Claims Database, 2010-2012.23
Note: 12-month and 9-month cohorts were derived from the 6-month cohort.
SE = standard error.
Switching Patterns
Of prevalent atrial fibrillation patients treated with dabigatran with at least 6 months of follow-up, 93.8% (n = 2,713) used dabigatran as the only oral anticoagulant, whereas the remainder 6.2% (n = 180) filled prescriptions for at least 1 other oral anticoagulant during the follow-up period (Table 2). Of patients who switched, 97.2% (n = 175) switched to warfarin, while 2.8% (n = 5) initiated rivaroxaban (Table 3). The average time to switch to a second agent was 61.1 days (standard error [SE] = 48.8 days). The percentages of patients who switched to other anticoagulants and the average time to a switch increased as the follow-up period was extended.
TABLE 2.
Distribution of Switchers and Nonswitchers Among Individuals with Atrial Fibrillation
| Prevalent Atrial Fibrillation | Incident Atrial Fibrillation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up Period | 6 Months | 9 Months | 12 Months | 6 Months | 9 Months | 12 Months | |||||
| Number of patients | 2,893 | 1,713 | 768 | 408 | 238 | 96 | |||||
| Nonswitchers, n (%) | 2,713 (93.8) | 1,588 (92.7) | 687 (89.5) | 364 (89.2) | 213 (89.5) | 90 (93.8) | |||||
| Used 2 agents, n (%) | 180 (6.2) | 125 (7.3) | 80 (10.4) | 44 (10.8) | 25 (10.5) | 6 (6.3) | |||||
| Used 3 agents, n (%) | — | — | 1 (0.1) | — | — | — | |||||
Source: IMS Health LifeLink Health Plan Claims Database, 2010-2012.23
TABLE 3.
Adherence and Persistence Among Switchers (Used More than 1 Anticoagulant During the Follow-Up Period) of Atrial Fibrillation Patients
| Follow-up Period | Prevalent Cohort | Incident Cohort | |||||
|---|---|---|---|---|---|---|---|
| 6 Months | 9 Months | 12 Months | 6 Months | 9 Months | 12 Months | ||
| Number of patients, n | 180 | 125 | 81 | 44 | 25 | 6 | |
| Switching patterns | |||||||
| Average time to first switch, mean (SE) | 61.1 (48.8) | 92.2 (82.5) | 126.4 (114.6) | 74.09 (51.4) | 87.58 (66.3) | 102.0 (84.0) | |
| Drug switched, n (%) | Warfarin | 175 (97.2) | 119 (95.2) | 73 (90.1) | 43 (97.7) | 25 (100) | 6 (100) |
| Rivaroxaban | 5 (2.8) | 6 (4.8) | 8 (9.9) | 1 (2.3) | — | — | |
| Adherence to anticoagulants | |||||||
| MPR analysis | Mean MPR, mean (SE) | 0.84 (0.21) | 0.78 (0.24) | 0.75 (0.27) | 0.82 (0.23) | 0.79 (0.24) | 0.81 (0.30) |
| Adherent (MPR ≥ 0.80), n (%) | 123 (68.3) | 65 (52.0) | 42 (51.9) | 31 (70.5) | 15 (60.0) | 4 (66.7) | |
| Nonadherent (MPR < 0.80), n (%) | 57 (31.7) | 60 (48.0) | 39 (48.1) | 13 (29.5) | 10 (40.0) | 2 (33.3) | |
| Persistence to anticoagulants | |||||||
| Main analysis | |||||||
| Gap = 60 days | Nonpersistent, n (%) | 38 (21.1) | 55 (44.0) | 38 (46.9) | 8 (18.2) | 12 (48.0) | 3 (50.0) |
| Sensitivity analyses | |||||||
| Gap = 15 days | Nonpersistent, n (%) | 112 (62.2) | 111 (88.8) | 62 (76.5) | 27 (61.4) | 22 (88.0) | 5 (83.3) |
| Gap = 30 days | Nonpersistent, n (%) | 77 (42.8) | 89 (71.2) | 50 (61.7) | 18 (40.9) | 17 (68.0) | 4 (66.7) |
| Gap = 90 days | Nonpersistent, n (%) | 17 (9.4) | 16 (12.8) | 29 (35.8) | 5 (11.4) | 9 (36.0) | 3 (50.0) |
Source: IMS Health LifeLink Health Plan Claims Database, 2010-2012.23
MPR = medication possession ratio; SE = standard error.
Adherence and Persistence
For patients with prevalent atrial fibrillation using dabigatran alone (n = 2,713), the mean MPR was 0.73 (SE = 0.30). Forty-one percent (n = 1,120) were nonadherent with dabigatran, and 31.5% (n = 854) had gaps of 60 days or greater (Table 4). The rates of nonadherence and nonpersistence increased with the follow-up period. Among those observed for 9 months who used dabigatran alone, mean MPR was 0.69 (SE = 0.33), while among those observed for 12 months, the mean MPR was 0.65 (SE = 0.35). Rates of nonadherence were 46.7% (n = 741) among the 9-month cohort and 49.2% (n = 338) among the 12-month cohort, whereas 48.0% (n = 763) of the 9-month cohort and 48.5% (n = 333) of the 12-month cohort had gaps of 60 days and greater during follow-up. Table 5 shows the baseline characteristics of adherent and nonadherent patients. Adherent subjects had higher average age and higher average CHADS2 score at baseline in all 3 cohorts. A Kaplan-Meier survival curve showing patients’ persistence over 12 months is shown in Figure 2.
TABLE 4.
Adherence and Persistence to Dabigatran Among Nonswitchers (Used Only Dabigatran During the Follow-Up Period) of Atrial Fibrillation Patients
| Follow-up Period | Prevalent Cohort | Incident Cohort | |||||
|---|---|---|---|---|---|---|---|
| 6 Months | 9 Months | 12 Months | 6 Months | 9 Months | 12 Months | ||
| Number of patients | 2,713 | 1,588 | 687 | 364 | 213 | 90 | |
| Adherence to dabigatran | |||||||
| MPR analysis | Mean MPR, mean (SE) | 0.73 (0.30) | 0.69 (0.33) | 0.65 (0.35) | 0.72 (0.31) | 0.67 (0.33) | 0.59 (0.35) |
| Adherent (MPR ≥ 0.80), n (%) | 1,593 (58.7) | 847 (53.3) | 349 (50.8) | 216 (59.3) | 109 (51.2) | 37 (41.1) | |
| Nonadherent (MPR < 0.80), n (%) | 1,120 (41.3) | 741 (46.7) | 338 (49.2) | 148 (40.7) | 104 (48.8) | 53 (58.9) | |
| Persistence to dabigatran | |||||||
| Main analysis | |||||||
| Gap = 60 days | Nonpersistent, n (%) | 854 (31.5) | 763 (48.0) | 333 (48.5) | 130 (35.7) | 95 (44.6) | 52 (57.8) |
| Sensitivity analyses | |||||||
| Gap = 15 days | Nonpersistent, n (%) | 1,551 (57.2) | 1,317 (82.9) | 495 (72.1) | 212 (58.2) | 144 (67.6) | 71 (78.9) |
| Gap = 30 days | Nonpersistent, n (%) | 1,203 (44.3) | 1,024 (64.5) | 417 (60.7) | 162 (44.5) | 115 (54.0) | 58 (64.4) |
| Gap = 90 days | Nonpersistent, n (%) | 600 (22.1) | 344 (21.7) | 283 (41.2) | 94 (25.8) | 82 (38.5) | 46 (51.1) |
Source: IMS Health LifeLink Health Plan Claims Database, 2010-2012.23
MPR = medication possession ratio; SE = standard error.
TABLE 5.
Patient Characteristics of Prevalent Atrial Fibrillation Patients Who Were Nonswitchers, Comparing Adherent Patients to Nonadherent Patients
| Follow-up Period | Adherent (MPR ≥ 0.80) | Nonadherent (MPR < 0.80) | |||||
|---|---|---|---|---|---|---|---|
| 6 Months | 9 Months | 12 Months | 6 Months | 9 Months | 12 Months | ||
| Number of patients | 1,593 | 847 | 349 | 1,120 | 741 | 338 | |
| Age, mean (SE) | 64.7 (11.1) | 65.3 (11.0) | 65.2 (11.5) | 60.5 (11.8) | 60.4 (11.9) | 60.4 (11.8) | |
| Age, n (%) | |||||||
| 18-44 | 41 (2.6) | 18 (2.1) | 8 (2.3) | 69 (6.2) | 45 (6.1) | 24 (7.1) | |
| 45-64 | 876 (55.0) | 455 (53.7) | 191 (54.7) | 744 (66.4) | 500 (67.5) | 225 (66.6) | |
| 65-74 | 357 (22.4) | 193 (22.8) | 76 (21.8) | 166 (14.8) | 104 (14.0) | 49 (14.5) | |
| 75+ | 319 (20.0) | 181 (21.4) | 74 (21.2) | 141 (12.6) | 92 (12.4) | 40 (11.8) | |
| Gender, n (%) | |||||||
| Male | 1,085 (68.1) | 585 (69.1) | 248 (71.1) | 802 (71.6) | 544 (73.4) | 252 (74.6) | |
| Female | 508 (31.9) | 262 (30.9) | 101 (28.9) | 318 (28.4) | 197 (26.6) | 86 (25.4) | |
| Region of residence, n (%) | |||||||
| East | 147 (9.2) | 77 (9.1) | 39 (11.2) | 130 (11.6) | 75 (10.1) | 42 (12.4) | |
| Midwest | 485 (30.4) | 278 (32.8) | 122 (35.0) | 356 (31.8) | 241 (32.5) | 128 (37.9) | |
| South | 780 (49.0) | 394 (46.5) | 143 (41.0) | 526 (47.0) | 351 (47.4) | 129 (38.2) | |
| West | 181 (11.4) | 98 (11.6) | 45 (12.9) | 108 (9.6) | 74 (10.0) | 39 (11.5) | |
| CHADS2, mean (SE) | 1.62 (1.11) | 1.63 (1.11) | 1.63 (1.09) | 1.27 (1.11) | 1.25 (1.06) | 1.25 (1.08) | |
| CHADS2, n (%) | |||||||
| 0 | 230 (14.4) | 124 (14.6) | 50 (14.3) | 291 (26.0) | 186 (25.1) | 84 (24.9) | |
| 1 | 576 (36.2) | 294 (34.7) | 118 (33.8) | 444 (39.6) | 304 (41.0) | 145 (42.9) | |
| 2 | 478 (30.0) | 260 (30.7) | 115 (33.0) | 248 (22.1) | 168 (22.7) | 70 (20.7) | |
| 3 | 215 (13.5) | 120 (14.2) | 49 (14.0) | 84 (7.5) | 54 (7.3) | 25 (7.4) | |
| 4 | 71 (4.5) | 38 (4.5) | 12 (3.4) | 39 (3.5) | 24 (3.2) | 10 (3.0) | |
| 5 | 22 (1.4) | 11 (1.3) | 5 (1.4) | 12 (1.1) | 4 (0.5) | 3 (0.9) | |
| 6 | 1 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 1 (0.1) | 1 (0.3) | |
Source: IMS Health LifeLink Health Plan Claims Database, 2010-2012.23
MPR = medication possession ratio; SE = standard error.
FIGURE 2.
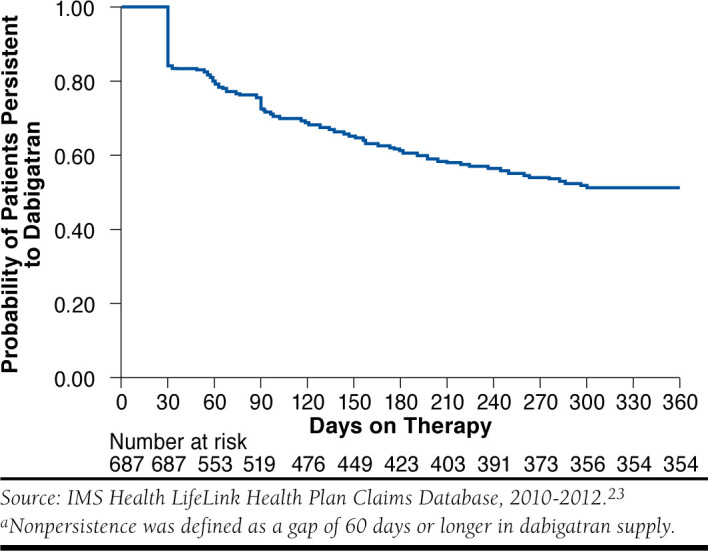
Kaplan-Meier Survival Curve of Prevalent Atrial Fibrillation Patients’ Persistence to Dabigatran in 1 Yeara
Among those exposed to multiple anticoagulants (switchers, Table 3), the mean MPR for anticoagulants was 0.84 (SE = 0.21). Of all prevalent atrial fibrillation patients, 31.7% (n = 57) were nonadherent to anticoagulants within the 6-month follow-up period, while 21.1% (n = 38) who were nonpersistent had gaps of 60 days or greater. Similarly, the rate of nonadherence and nonpersistence increased with a longer follow-up period.
Rates of adherence and persistence were similar when analyses were limited to patients with incident atrial fibrillation (Tables 3 and 4).
Discussion
Using individual-level claims data from 2010 to 2012, we found a mean MPR of 0.73 among patients with prevalent atrial fibrillation using dabigatran within 6 months of initiating therapy. Using an 0.80 cutoff point, 2 in 5 new dabigatran users were nonadherent. On average, the adherent patients were of older age and had higher baseline risk for stroke. Mean MPRs decreased and rates of nonadherence increased as the length of follow-up increased, with nearly half of new dabigatran users being nonadherent at 1 year. Approximately 6% of patients switched to other anticoagulants within 6 months, the majority of whom initiated warfarin. These findings are important because novel oral anticoagulants represent an important new treatment option for atrial fibrillation and, increasingly, other common and costly thromboembolic disorders, yet little is known about individuals’ real-world adherence to these products.
At least 3 studies have examined adherence and persistence to dabigatran.7-9 The first used data from a large pharmacy benefit manager and identified a nonpersistence rate of 40% (defined as a gap of 30 days or more in prescriptions) during 180-day follow-up period among patients with or without exposure to warfarin in the 180 days prior to initiating dabigatran.7 Moreover, the study reported a mean proprortion of days covered (PDC) of 0.67 for the warfarin-naïve cohort (no exposure to warfarin within 180 days prior to initiating dabigatran). We observed a similar nonadherence rate (44.3%) and mean MPR (mean MPR = 0.73) in our study. The second investigation, which followed a group of dabigatran users at a single medical center by administering questionnaires, identified that 70% of 92 recruited patients remained on dabigatran over 3-12 months.8 Another study evaluated adherence to dabigatran using medical and pharmacy records at a medical center and affiliated clinic.9 Of all 159 eligible subjects, 43% had an MPR < 0.80 and the mean MPR was 0.63. Although this study measured adherence by prescriptions picked up at a local pharmacy and validated through orders of medical records, a different methodological approach from ours, the rate of nonadherence (MPR < 0.80) was similar to our findings. One possible explanation for the higher mean MPR observed in our study was the inability to exclude treatment discontinuation initiated by prescribers. In contrast to the prior studies, we focused on new users of anticoagulants and followed patients for up to 1 year, a longer period compared with the previous analyses. Moreover, our study involved a national sample of users larger than a single center investigation, which is more generalizable.
We assessed adherence to dabigatran for up to 1 year, which is clinically relevant since anticoagulation is recommended for longterm use among atrial fibrillation patients.17,18 This longer follow-up period also allowed us to identify increases in nonadherence and nonpersistence to dabigatran/anticoagulants over time. This has been identified as a problem for many chronic therapies,19 including cardiovascular treatments,20 and reflects one of the perennial challenges regarding adherence. For example, in a retrospective cohort study of nonvalvular atrial fibrillation patients aged 18 years and above, the percentages of patients with a PDC of 80% or greater for twice-daily metoprolol and carvedilol were 50% and 53%, respectively.21 These proportions were higher (62% and 63%, respectively) for those who took metoprolol and carvedilol once daily. Future investigations to identify associated patient or physician characteristics that lead to discontinuation of therapy are needed.
Prior studies suggested that poor adherence to anticoagulant therapies was associated with poor clinical outcomes. For example, the IN-RANGE study, a prospective cohort study that followed patients at anticoagulation clinics, found a significant association between poor adherence to warfarin and underanticoagulation measured as the international normalized ratio (INR) less than the lower limit of the target range.22 Future studies should explore the predictors of nonadherence and test interventions to improve adherence to novel anticoagulants.
Limitations
Our study has some limitations. Although there are 3 novel oral anticoagulants available on the market, we limited our analyses to dabigatran since it was the first agent to receive U.S. market approval and the complicated dosing of warfarin precluded comparison of warfarin vs. dabigatran adherence. The data excluded some clinical variables of interest and thus do not allow for us to quantify the degree to which observed nonadherence was due to patient, physician, or health-system factors. In some cases, nonuse may have represented clinically appropriate treatment discontinuations due to adverse effects, such as bleeding. Our estimates presume that patients take all the medications they fill rather than stockpiling them, an assumption that is inherent within all adherence analyses using administrative claims data. Our analyses were limited to patients with continuous coverage and thus may underestimate the true rates of nonadherence among a broader population. Finally, as with most adherence studies that incorporate a measure of MPR, we used a conventional cutoff point of 0.80 to classify patients as adherent or not. The reasonableness of this cutoff point varies based on a variety of factors including disease cohorts, classes of medications administered, and endpoints of interest.16
Conclusions
Our findings suggest that 2 in 5 patients with atrial fibrillation may not adhere to dabigatran after 6 months, and up to one half of patients are poorly adherent 1 year out from the initiation of therapy. These findings were similar to those seen in prior analyses.7-9 Given the importance of adherence to anticoagulation for the prevention and treatment of thromboembolism, future studies are needed to understand the underlying reasons for the observed nonadherence and how to mitigate them. Moreover, investigations of associations between adherence and relevant clinical outcomes are needed to fully understand the degree to which the safety and effectiveness of dabigatran and other novel oral anticoagulants are affected by such patterns of utilization.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-75. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kanne WBl.. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147(9):1561-64. [PubMed] [Google Scholar]
- 3.De Caterina R, Husted S, Wallentin L, et al. ; Coordinating Committee. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC Working Group on Thrombosis-Task Force on Anticoagulants in Heart Disease position paper. J Am Coll Cardiol. 2012;59(16):1413-25. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-51. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Bajorek B.. New oral anticoagulants in practice: pharmacological and practical considerations. Am J Cardiovasc Drugs. 2014;14(3):175-89. [DOI] [PubMed] [Google Scholar]
- 6.Claxton AJ, Cramer J, Pierce C.. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8): 1296-310. [DOI] [PubMed] [Google Scholar]
- 7.Tsai K, Erickson SC, Yang J, Harada AS, Solow BK, Lew HC.. Adherence, persistence, and switching patterns of dabigatran etexilate. Am J Manag Care. 2013;19(9):e325-32. [PubMed] [Google Scholar]
- 8.Thorne K, Jayathissa S, Dee S, et al. Adherence and outcomes of patients prescribed dabigatran (Pradaxa) in routine clinical practice. Intern Med J. 2014;44(3):261-55. [DOI] [PubMed] [Google Scholar]
- 9.Culter TW, Chuang A, Huynh TD, et al. A retrospective descriptive analysis of patient adherence to dabigatran at a large academic medical center. J Manag Care Spec Pharm. 2014;20(10):1028-34. Available at: http://www.amcp.org/JMCP/2014/October/18587/1033.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulman S, Kearon C, Kakkar AK, et al. ; the RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-52. [DOI] [PubMed] [Google Scholar]
- 11.Motheral BR, Fairman KA.. The use of claims database for outcome research: rationale, challenges, and strategies. Clin Ther. 1997;19(2):346-66. [DOI] [PubMed] [Google Scholar]
- 12.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S.. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1): 141-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ.. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864-70. [DOI] [PubMed] [Google Scholar]
- 14.Rothendler JA, Rose AJ, Reisman JI, Berlowitz DR, Kazis LE.. Choices in the use of ICD-9 codes to identify stroke risk factors can affect the apparent population-level risk factor prevalence and distribution of CHADS2 scores. Am J Cardiovasc Dis. 2012;2(3):184-91. [PMC free article] [PubMed] [Google Scholar]
- 15.Tirschwell DL, Longstreth WT Jr.. Validating administrative data in stroke research. Stroke. 2002;33(10):2465-70. [DOI] [PubMed] [Google Scholar]
- 16.Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC.. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303-10. [DOI] [PubMed] [Google Scholar]
- 17.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-80. [DOI] [PubMed] [Google Scholar]
- 18.Wann LS, Curtis AB, Ellenbogen KA, et al. ; American College of Cardiology Foundation/American Heart Association Task Force. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123(10):1144-50. [DOI] [PubMed] [Google Scholar]
- 19.Sabate E. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization, 2003. Available at: http://whqlibdoc.who.int/publications/2003/9241545992.pdf. Accessed September 18, 2015.
- 20.Naderi SH, Bestwick JP, Wald DS.. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125(9):882-87.e1. [DOI] [PubMed] [Google Scholar]
- 21.Burton T, Fan Y, Kwong WJ.. Dosing frequency and adherence in patients with nonvalvular atrial fibrillation. Am J Pharm Benefits. 2014;6(2):e31-e40. [Google Scholar]
- 22.Kimmel SE, Chen Z, Price M, et al.. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Arch Intern Med. 2007;167(3):229-35. [DOI] [PubMed] [Google Scholar]
- 23.IMS Health Incorporated or its affiliates. LifeLink Health Plan claims data user guide & data dictionary. 2012. [Google Scholar]